Effective non-viral delivery of siRNA to acute myeloid leukemia cells with lipid-substituted polyethylenimines
- PMID: 22952927
- PMCID: PMC3432090
- DOI: 10.1371/journal.pone.0044197
Effective non-viral delivery of siRNA to acute myeloid leukemia cells with lipid-substituted polyethylenimines
Abstract
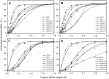
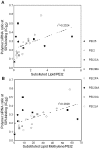
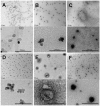
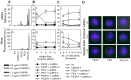
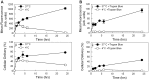
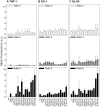
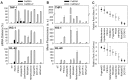
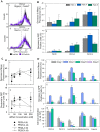
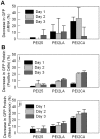
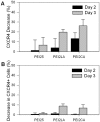
Use of small interfering RNA (siRNA) is a promising approach for AML treatment as the siRNA molecule can be designed to specifically target proteins that contribute to aberrant cell proliferation in this disease. However, a clinical-relevant means of delivering siRNA molecules must be developed, as the cellular delivery of siRNA is problematic. Here, we report amphiphilic carriers combining a cationic polymer (2 kDa polyethyleneimine, PEI2) with lipophilic moieties to facilitate intracellular delivery of siRNA to AML cell lines. Complete binding of siRNA by the designed carriers was achieved at a polymer:siRNA ratio of ≈ 0.5 and led to siRNA/polymer complexes of ≈ 100 nm size. While the native PEI2 did not display cytotoxicity on AML cell lines THP-1, KG-1 and HL-60, lipid-modification on PEI2 slightly increased the cytotoxicity, which was consistent with increased interaction of polymers with cell membranes. Cellular delivery of siRNA was dependent on the nature of lipid substituent and the extent of lipid substitution, and varied among the three AML cell lines used. Linoleic acid-substituted polymers performed best among the prepared polymers and gave a siRNA delivery equivalent to better performing commercial reagents. Using THP-1 cells and a reporter (GFP) and an endogenous (CXCR4) target, effective silencing of the chosen targets was achieved with 25 to 50 nM of siRNA concentrations, and without adversely affecting subsequent cell growth. We conclude that lipid-substituted PEI2 can serve as an effective delivery of siRNA to leukemic cells and could be employed in molecular therapy of leukemia.
Conflict of interest statement
Figures




References
-
- Cancer Facts & Figures (2012) from American Cancer Society web site. Available: http://www.cancer.org/Research/CancerFactsFigures/index. Accessed June 30, 2012.
-
- Sekeres MA (2008) Treatment of older adults with acute myeloid leukemia: state of the art and current perspectives. Haematolog 93: 1769–1772. - PubMed
-
- Kasper GJ, Zwan CM (2007) Pediatric acute myeloid leukemia:towards high quality cure of all patients. Haematolog 92: 1519–1532. - PubMed
-
- Löwenberg B, Downing JR, Burnett A (2000) Acute myeloid leukemia. NEJM 341: 1051–1063. - PubMed
-
- Cioca DP, Aoki Y, Kiyosawa K (2003) RNA interference is a functional pathway with therapeutic potential in human myeloid leukemia cell lines. Cancer Gene Ther 10: 125–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

