The PICALM protein plays a key role in iron homeostasis and cell proliferation
- PMID: 22952941
- PMCID: PMC3431333
- DOI: 10.1371/journal.pone.0044252
The PICALM protein plays a key role in iron homeostasis and cell proliferation
Abstract
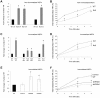
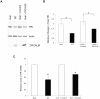
The ubiquitously expressed phosphatidylinositol binding clathrin assembly (PICALM) protein associates with the plasma membrane, binds clathrin, and plays a role in clathrin-mediated endocytosis. Alterations of the human PICALM gene are present in aggressive hematopoietic malignancies, and genome-wide association studies have recently linked the PICALM locus to late-onset Alzheimer's disease. Inactivating and hypomorphic Picalm mutations in mice cause different degrees of severity of anemia, abnormal iron metabolism, growth retardation and shortened lifespan. To understand PICALM's function, we studied the consequences of PICALM overexpression and characterized PICALM-deficient cells derived from mutant fit1 mice. Our results identify a role for PICALM in transferrin receptor (TfR) internalization and demonstrate that the C-terminal PICALM residues are critical for its association with clathrin and for the inhibitory effect of PICALM overexpression on TfR internalization. Murine embryonic fibroblasts (MEFs) that are deficient in PICALM display several characteristics of iron deficiency (increased surface TfR expression, decreased intracellular iron levels, and reduced cellular proliferation), all of which are rescued by retroviral PICALM expression. The proliferation defect of cells that lack PICALM results, at least in part, from insufficient iron uptake, since it can be corrected by iron supplementation. Moreover, PICALM-deficient cells are particularly sensitive to iron chelation. Taken together, these data reveal that PICALM plays a critical role in iron homeostasis, and offer new perspectives into the pathogenesis of PICALM-associated diseases.
Conflict of interest statement
Figures




References
-
- Meyerholz A, Hinrichsen L, Groos S, Esk P-C, Brandes G, et al. (2005) Effect of clathrin assembly lymphoid myeloid leukemia protein depletion on clathrin coat formation. Traffic 6: 1225–1234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

