Pro-angiogenic and anti-inflammatory regulation by functional peptides loaded in polymeric implants for soft tissue regeneration
- PMID: 22953721
- PMCID: PMC3542873
- DOI: 10.1089/ten.TEA.2012.0158
Pro-angiogenic and anti-inflammatory regulation by functional peptides loaded in polymeric implants for soft tissue regeneration
Abstract
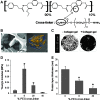
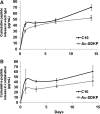
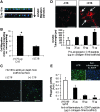
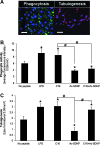
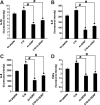
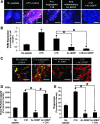
Inflammation and angiogenesis are inevitable in vivo responses to biomaterial implants. Continuous progress has been made in biomaterial design to improve tissue interactions with an implant by either reducing inflammation or promoting angiogenesis. However, it has become increasingly clear that the physiological processes of inflammation and angiogenesis are interconnected through various molecular mechanisms. Hence, there is an unmet need for engineering functional tissues by simultaneous activation of pro-angiogenic and anti-inflammatory responses to biomaterial implants. In this work, the modulus and fibrinogen adsorption of porous scaffolds were tuned to meet the requirements (i.e., ~100 kPa and ~10 nm, respectively), for soft tissue regeneration by employing tyrosine-derived combinatorial polymers with polyethylene glycol crosslinkers. Two types of functional peptides (i.e., pro-angiogenic laminin-derived C16 and anti-inflammatory thymosin β4-derived Ac-SDKP) were loaded in porous scaffolds through collagen gel embedding so that peptides were released in a controlled fashion, mimicking degradation of the extracellular matrix. The results from (1) in vitro coculture of human umbilical vein endothelial cells and human blood-derived macrophages and (2) in vivo subcutaneous implantation revealed the directly proportional relationship between angiogenic activities (i.e., tubulogenesis and perfusion capacity) and inflammatory activities (i.e., phagocytosis and F4/80 expression) upon treatment with either type of peptide. Interestingly, cotreatment with both types of peptides upregulated the angiogenic responses, while downregulating the inflammatory responses. Also, anti-inflammatory Ac-SDKP peptides reduced production of pro-inflammatory cytokines (i.e., interleukin [IL]-1β, IL-6, IL-8, and tumor necrosis factor alpha) even when treated in combination with pro-angiogenic C16 peptides. In addition to independent regulation of angiogenesis and inflammation, this study suggests a promising approach to improve soft tissue regeneration (e.g., blood vessel and heart muscle) when inflammatory diseases (e.g., ischemic tissue fibrosis and atherosclerosis) limit the regeneration process.
Figures
References
-
- Bailey L.O. Washburn N.R. Simon C.G. Chan E.S. Wang F.W. Quantification of inflammatory cellular responses using real-time polymerase chain reaction. J Biomed Mater Res A. 2004;69A:305. - PubMed
-
- Hu W.J. Eaton J.W. Tang L.P. Molecular basis of biomaterial-mediated foreign body reactions. Blood. 2001;98:1231. - PubMed
-
- Ratner B.D. Gladhill K.W. Horbett T.A. Analysis of in vitro enzymatic and oxidative degradation of polyurethanes. J Biomed Mater Res. 1988;22:509. - PubMed
-
- Silva M.M. Cyster L.A. Barry J.J. Yang X.B. Oreffo R.O. Grant D.M., et al. The effect of anisotropic architecture on cell and tissue infiltration into tissue engineering scaffolds. Biomaterials. 2006;27:5909. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

