Prevention of pneumococcal diseases in the post-seven valent vaccine era: a European perspective
- PMID: 22954038
- PMCID: PMC3462147
- DOI: 10.1186/1471-2334-12-207
Prevention of pneumococcal diseases in the post-seven valent vaccine era: a European perspective
Abstract
Background: The burden of invasive pneumococcal disease in young children decreased dramatically following introduction of the 7-valent pneumococcal conjugate vaccine (PCV7). The epidemiology of S. pneumoniae now reflects infections caused by serotypes not included in PCV7. Recently introduced higher valency pneumococcal vaccines target the residual burden of invasive and non-invasive infections, including those caused by serotypes not included in PCV7. This review is based on presentations made at the European Society of Pediatric Infectious Diseases in June 2011.
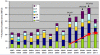
Discussion: Surveillance data show increased circulation of the non-PCV7 vaccine serotypes 1, 3, 6A, 6C, 7 F and 19A in countries with routine vaccination. Preliminary evidence suggests that broadened serotype coverage offered by higher valency vaccines may be having an effect on invasive disease caused by some of those serotypes, including 19A, 7 F and 6C. Aetiology of community acquired pneumonia remains a difficult clinical diagnosis. However, recent reports indicate that pneumococcal vaccination has reduced hospitalisations of children for vaccine serotype pneumonia. Variations in serotype circulation and occurrence of complicated and non-complicated pneumonia caused by non-PCV7 serotypes highlight the potential of higher valency vaccines to decrease the remaining burden. PCVs reduce nasopharyngeal carriage and acute otitis media (AOM) caused by vaccine serotypes. Recent investigations of the interaction between S. pneumoniae and non-typeable H. influenzae suggest that considerable reduction in severe, complicated AOM infections may be achieved by prevention of early pneumococcal carriage and AOM infections. Extension of the vaccine serotype spectrum beyond PCV7 may provide additional benefit in preventing the evolution of AOM. The direct and indirect costs associated with pneumococcal disease are high, thus herd protection and infections caused by non-vaccine serotypes both have strong effects on the cost effectiveness of pneumococcal vaccination. Recent evaluations highlight the public health significance of indirect benefits, prevention of pneumonia and AOM and coverage of non-PCV7 serotypes by higher valency vaccines.
Summary: Routine vaccination has greatly reduced the burden of pneumococcal diseases in children. The pneumococcal serotypes present in the 7-valent vaccine have greatly diminished among disease isolates. The prevalence of some non-vaccine serotypes (e.g. 1, 7 F and 19A) has increased. Pneumococcal vaccines with broadened serotype coverage are likely to continue decreasing the burden of invasive disease, and community acquired pneumonia in children. Further reductions in pneumococcal carriage and increased prevention of early AOM infections may prevent the evolution of severe, complicated AOM. Evaluation of the public health benefits of pneumococcal conjugate vaccines should include consideration of non-invasive pneumococcal infections, indirect effects of vaccination and broadened serotype coverage.
Figures

References
-
- Robinson KA, Baughman W, Rothrock G, Barrett NL, Pass M, Lexau C, Damaske B, Stefonek K, Barnes B, Patterson J, Zell ER, Schuchat A, Whitney CG. Active Bacterial Core Surveillance (ABCs)/Emerging Infections Program Network. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998: Opportunities for prevention in the conjugate vaccine era. JAMA. 2001;285(13):1729–1735. doi: 10.1001/jama.285.13.1729. - DOI - PubMed
-
- Eskola J, Takala AK, Kela E, Pekkanen E, Kalliokoski R, Leinonen M. Epidemiology of invasive pneumococcal infections in children in Finland. JAMA. 1992;16(268):3323–3327. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

