The role of protease-activated receptor-2 on pulmonary neutrophils in the innate immune response to cockroach allergen
- PMID: 22954301
- PMCID: PMC3724482
- DOI: 10.1186/1476-9255-9-32
The role of protease-activated receptor-2 on pulmonary neutrophils in the innate immune response to cockroach allergen
Abstract
Background: Serine proteases in German cockroach (GC) have been shown to mediate allergic airway inflammation through the activation of protease activated receptor (PAR)-2. Neutrophils play an important role in regulating the innate immune response, and are recruited into the airways following GC frass exposure. As such, we investigated the role of PAR-2 in airway neutrophil recruitment, activation and cytokine production following allergen exposure.
Methods: Wild type and PAR-2-deficient mice were administered a single intratracheal instillation of PBS or GC frass and neutrophil recruitment, expression of PAR-2, CD80, CD86, and MHC class II were assessed by flow cytometry and levels of tumor necrosis factor (TNF)α was assessed by ELISA. Uptake of AlexaFluor 405-labeled GC frass by neutrophils was performed by flow cytometry.
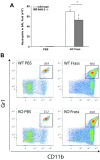
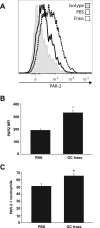
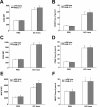
Results: Neutrophil recruitment in the lung and airways following GC frass exposure was significantly decreased in PAR-2-deficient mice compared to wild type mice. GC frass exposure increased the level of PAR-2 on pulmonary neutrophils and increased numbers of PAR-2-positive neutrophils were found in the lungs; however PAR-2 did not play a role in meditating allergen uptake. Comparing wild type and PAR-2-deficient mice, we found that a single exposure to GC frass increased levels of CD80 and CD86 on pulmonary neutrophils, an effect which was independent of PAR-2 expression. Neutrophils isolated from the whole lungs of naïve PAR-2-deficient mice treated ex vivo with GC frass produced significantly less TNFα than in similarly treated wild type neutrophils. Lastly, neutrophils were isolated from the bronchoalveolar lavage fluid of wild type and PAR-2-deficient mice following a single intratracheal exposure to GC frass. Airway neutrophils from PAR-2-deficient mice released substantially decreased levels of TNFα, suggesting a role for PAR-2 in neutrophil-derived cytokine production.
Conclusions: Together these data suggest PAR-2 expression can be upregulated on lung neutrophils following allergen exposure and the consequence is altered release of TNFα which could drive the early innate immune response.
Figures


Similar articles
-
Protease-activated receptor 2 activation of myeloid dendritic cells regulates allergic airway inflammation.Respir Res. 2011 Sep 21;12(1):122. doi: 10.1186/1465-9921-12-122. Respir Res. 2011. PMID: 21936897 Free PMC article.
-
Mucosal sensitization to German cockroach involves protease-activated receptor-2.Respir Res. 2010 May 24;11(1):62. doi: 10.1186/1465-9921-11-62. Respir Res. 2010. PMID: 20497568 Free PMC article.
-
German cockroach frass proteases modulate the innate immune response via activation of protease-activated receptor-2.J Innate Immun. 2010;2(5):495-504. doi: 10.1159/000317195. Epub 2010 Jun 26. J Innate Immun. 2010. PMID: 20588004 Free PMC article.
-
German cockroach proteases and protease-activated receptor-2 regulate chemokine production and dendritic cell recruitment.J Innate Immun. 2012;4(1):100-10. doi: 10.1159/000329132. Epub 2011 Aug 29. J Innate Immun. 2012. PMID: 21876326 Free PMC article.
-
Cockroach allergen exposure and risk of asthma.Allergy. 2016 Apr;71(4):463-74. doi: 10.1111/all.12827. Epub 2016 Feb 4. Allergy. 2016. PMID: 26706467 Free PMC article. Review.
Cited by
-
Bronchoalveolar lavage cell profiles and proteins concentrations can be used to phenotype extrinsic allergic alveolitis patients.Multidiscip Respir Med. 2019 Mar 12;14:13. doi: 10.1186/s40248-019-0175-6. eCollection 2019. Multidiscip Respir Med. 2019. PMID: 30911386 Free PMC article.
-
Evaluation on potential contributions of protease activated receptors related mediators in allergic inflammation.Mediators Inflamm. 2014;2014:829068. doi: 10.1155/2014/829068. Epub 2014 Apr 30. Mediators Inflamm. 2014. PMID: 24876677 Free PMC article. Review.
-
Airborne indoor allergen serine proteases and their contribution to sensitisation and activation of innate immunity in allergic airway disease.Eur Respir Rev. 2024 Apr 24;33(172):230126. doi: 10.1183/16000617.0126-2023. Print 2024 Apr 30. Eur Respir Rev. 2024. PMID: 38657996 Free PMC article. Review.
-
The Role of Heat Shock Protein 70 kDa in Asthma.J Asthma Allergy. 2021 Jan 5;13:757-772. doi: 10.2147/JAA.S288886. eCollection 2020. J Asthma Allergy. 2021. PMID: 33447061 Free PMC article. Review.
-
Stenotrophomonas maltophilia Serine Protease StmPr1 Induces Matrilysis, Anoikis, and Protease-Activated Receptor 2 Activation in Human Lung Epithelial Cells.Infect Immun. 2017 Nov 17;85(12):e00544-17. doi: 10.1128/IAI.00544-17. Print 2017 Dec. Infect Immun. 2017. PMID: 28893914 Free PMC article.
References
-
- Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med. 1999;160:1532–1539. - PubMed
-
- Ordonez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma: clinical and biological significance. Am J Respir Crit Care Med. 2000;161:1185–1190. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

