The human proton-coupled folate transporter: Biology and therapeutic applications to cancer
- PMID: 22954694
- PMCID: PMC3542225
- DOI: 10.4161/cbt.22020
The human proton-coupled folate transporter: Biology and therapeutic applications to cancer
Abstract
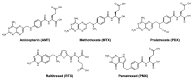
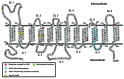
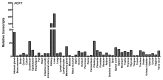
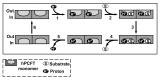
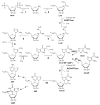
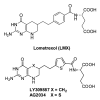
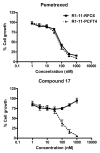
This review summarizes the biology of the proton-coupled folate transporter (PCFT). PCFT was identified in 2006 as the primary transporter for intestinal absorption of dietary folates, as mutations in PCFT are causal in hereditary folate malabsorption (HFM) syndrome. Since 2006, there have been major advances in understanding the mechanistic roles of critical amino acids and/or domains in the PCFT protein, many of which were identified as mutated in HFM patients, and in characterizing transcriptional control of the human PCFT gene. With the recognition that PCFT is abundantly expressed in human tumors and is active at pHs characterizing the tumor microenvironment, attention turned to exploiting PCFT for delivering novel cytotoxic antifolates for solid tumors. The finding that pemetrexed is an excellent PCFT substrate explains its demonstrated clinical efficacy for mesothelioma and non-small cell lung cancer, and prompted development of more PCFT-selective tumor-targeted 6-substituted pyrrolo[2,3-d]pyrimidine antifolates that derive their cytotoxic effects by targeting de novo purine nucleotide biosynthesis.
Figures

References
-
- Farber S. Some observations on the effect of folic acid antagonists on acute leukemia and other forms of incurable cancer. Blood. 1949;4:160–7. - PubMed
-
- Monahan BP, Allegra CJ. Antifolates. In: Chabner BA, Longo, D.L., ed. Cancer Chemotherapy and Biotherapy. Philadelphia, PA: Lippincott Williams and Wilkins, 2011:109-38.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
