Preliminary pharmacology of galactosylated chitosan/5-fluorouracil nanoparticles and its inhibition of hepatocellular carcinoma in mice
- PMID: 22954702
- PMCID: PMC3542231
- DOI: 10.4161/cbt.22001
Preliminary pharmacology of galactosylated chitosan/5-fluorouracil nanoparticles and its inhibition of hepatocellular carcinoma in mice
Abstract
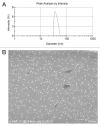
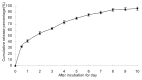
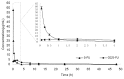
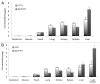
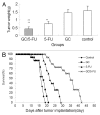
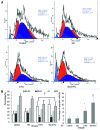
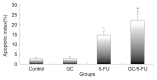
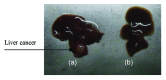
Biodegradable polymer nanoparticle drug delivery systems are characterized by targeted drug delivery, improved pharmacokinetic and biodistribution, enhanced drug stability and lowered side effects; these drug delivery systems are widely used for delivery of cytotoxic agents. The galactosylated chitosan (GC)/5-fluorouracil (5-FU) nanoparticle is a nanomaterial made by coupling GC, a polymer known to have the advantages described above, and 5-FU. The GC/5-FU nanoparticle is a sustained release system, it was showed that the peak time, half-life time, mean residence time (MRT) and area of under curve (AUC) of GC/5-FU were longer or more than those of the 5-FU group, but the maximum concentration (Cmax) was lower. The distribution of GC/5-FU in vivo revealed the greatest accumulation in the hepatic cancer tissues, and the hepatic cell was the target of the nanoparticles. Toxicology research showed that the toxicity of GC-5-FU was lower than that of 5-FU in mice. In vivo experiments showed that GC/5-FU can significantly inhibit tumor growth in an orthotropic liver cancer mouse model. GC/5-FU treatment can significantly lower the tumor weight and increase the survival time of mice when compared with 5-FU treatment alone. Flow cytometry and the TUNEL assay revealed that compared with 5-FU, GC/5-FU caused higher rates of G 0-G 1 arrest and apoptosis in hepatic cancer cells.
Figures

References
-
- Namikawa T, Fukudome I, Kitagawa H, Okabayashi T, Kobayashi M, Hanazaki K. Plasma diamine oxidase activity is a useful biomarker for evaluating gastrointestinal tract toxicities during chemotherapy with oral fluorouracil anti-cancer drugs in patients with gastric cancer. Oncology. 2012;82:147–52. doi: 10.1159/000336799. - DOI - PubMed
-
- Sakata Y, Komatsu Y, Takagi S, Saitoh S, Itoh T, Suzuki H, et al. Randomized controlled study of mitomycin C/carboquone/5-fluorouracil/OK-432 (MQ-F-OK) therapy and mitomycin C/5-fluorouracil/doxorubicin (FAM) therapy against advanced liver cancer. Cancer Chemother Pharmacol. 1989;23(Suppl):S9–12. doi: 10.1007/BF00647230. - DOI - PubMed
-
- Kanetaka K, Enjoji A, Furui J, Nagata Y, Fujioka H, Shiogama T, et al. The Nagasaki Study Group for Digestive Organ Cancer Chemotherapy Effects of Intermittent 5-Fluorouracil and Low-dose Cisplatin Therapy on Advanced and Recurrent Gastric Cancer. Anticancer Res. 2012;32:3495–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
