MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study
- PMID: 22954705
- PMCID: PMC4074206
- DOI: 10.1016/S1474-4422(12)70203-X
MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study
Abstract
Background: Whether endovascular stroke treatment improves clinical outcomes is unclear because of the paucity of data from randomised placebo-controlled trials. We aimed to establish whether MRI can be used to identify patients who are most likely to benefit from endovascular reperfusion.
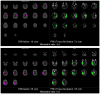
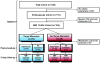
Methods: In this prospective cohort study we consecutively enrolled patients scheduled to have endovascular treatment within 12 h of onset of stroke at eight centres in the USA and one in Austria. Aided by an automated image analysis computer program, investigators interpreted a baseline MRI scan taken before treatment to establish whether the patient had an MRI profile (target mismatch) that suggested salvageable tissue was present. Reperfusion was assessed on an early follow-up MRI scan (within 12 h of the revascularisation procedure) and defined as a more than 50% reduction in the volume of the lesion from baseline on perfusion-weighted MRI. The primary outcome was favourable clinical response, defined as an improvement of 8 or more on the National Institutes of Health Stroke Scale between baseline and day 30 or a score of 0-1 at day 30. The secondary clinical endpoint was good functional outcome, defined as a modified Rankin scale score of 2 or less at day 90. Analyses were adjusted for imbalances in baseline predictors of outcome. Investigators assessing outcomes were masked to baseline data.
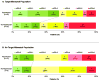
Findings: 138 patients were enrolled. 110 patients had catheter angiography and of these 104 had an MRI profile and 99 could be assessed for reperfusion. 46 of 78 (59%) patients with target mismatch and 12 of 21 (57%) patients without target mismatch had reperfusion after endovascular treatment. The adjusted odds ratio (OR) for favourable clinical response associated with reperfusion was 8·8 (95% CI 2·7-29·0) in the target mismatch group and 0·2 (0·0-1·6) in the no target mismatch group (p=0·003 for difference between ORs). Reperfusion was associated with increased good functional outcome at 90 days (OR 4·0, 95% CI 1·3-12·2) in the target mismatch group, but not in the no target mismatch group (1·9, 0·2-18·7).
Interpretation: Target mismatch patients who had early reperfusion after endovascular stroke treatment had more favourable clinical outcomes. No association between reperfusion and favourable outcomes was present in patients without target mismatch. Our data suggest that a randomised controlled trial of endovascular treatment for patients with the target mismatch profile is warranted.
Funding: National Institute for Neurological Disorders and Stroke.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
G. Albers has received consulting fees and expenses from Lundbeck for Steering Committee work and consulting fees from Concentric for serving on a Data Safely and Monitory Board. G Albers and R Bammer are equity shareholders in iSchemaView. G. Zaharchuk is a member of the Neuroradiology Advisory Board for GE Healthcare, and receives modest research funding support from GE Healthcare. Helmi Lutsep has received consulting fees and expenses from Concentric Medical for serving on the Executive Committee of the TREVO2 trial, from Co-Axia for serving on the DSMB of the SENTIS trial and from AGA Medical for serving on the Neurology Executive Committee of the RESPECT trial. All other authors report no conflicts of interest.
Figures
Comment in
-
Imaging and treatment response after ischaemic stroke.Lancet Neurol. 2012 Oct;11(10):838-9. doi: 10.1016/S1474-4422(12)70207-7. Epub 2012 Sep 4. Lancet Neurol. 2012. PMID: 22954706 No abstract available.
-
Selection of patients for intra-arterial therapy.Lancet Neurol. 2013 Mar;12(3):225. doi: 10.1016/S1474-4422(13)70018-8. Lancet Neurol. 2013. PMID: 23415562 No abstract available.
-
Selection of patients for intra-arterial therapy--authors' reply.Lancet Neurol. 2013 Mar;12(3):225-6. doi: 10.1016/S1474-4422(13)70019-X. Lancet Neurol. 2013. PMID: 23415563 No abstract available.
References
-
- Hirsch JA, Yoo AJ, Nogueira RG, Verduzco LA, Schwamm LH, Pryor JC, et al. Case volumes of intra-arterial and intravenous treatment of ischemic stroke in the USA. Journal of NeuroInterventional Surgery. 2009;1(1):27–31. - PubMed
-
- Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, et al. Mechanical Thrombectomy for Acute Ischemic Stroke: Final Results of the Multi MERCI Trial. Stroke. 2008;39(4):1205–12. - PubMed
-
- The Penumbra Pivotal Stroke Trial Investigators. The Penumbra Pivotal Stroke Trial: Safety and Effectiveness of a New Generation of Mechanical Devices for Clot Removal in Intracranial Large Vessel Occlusive Disease. Stroke. 2009;40(8):2761–8. - PubMed
-
- Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, et al. Safety and Efficacy of Mechanical Embolectomy in Acute Ischemic Stroke: Results of the MERCI Trial. Stroke. 2005;36(7):1432–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

