Signaling by FGF4 and FGF8 is required for axial elongation of the mouse embryo
- PMID: 22954964
- PMCID: PMC3481862
- DOI: 10.1016/j.ydbio.2012.08.017
Signaling by FGF4 and FGF8 is required for axial elongation of the mouse embryo
Abstract
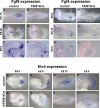
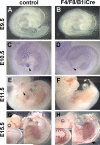
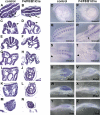
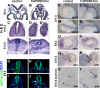
Fibroblast growth factor (FGF) signaling has been shown to play critical roles in vertebrate segmentation and elongation of the embryonic axis. Neither the exact roles of FGF signaling, nor the identity of the FGF ligands involved in these processes, has been conclusively determined. Fgf8 is required for cell migration away from the primitive streak when gastrulation initiates, but previous studies have shown that drastically reducing the level of FGF8 later in gastrulation has no apparent effect on somitogenesis or elongation of the embryo. In this study, we demonstrate that loss of both Fgf8 and Fgf4 expression during late gastrulation resulted in a dramatic skeletal phenotype. Thoracic vertebrae and ribs had abnormal morphology, lumbar and sacral vertebrae were malformed or completely absent, and no tail vertebrae were present. The expression of Wnt3a in the tail and the amount of nascent mesoderm expressing Brachyury were both severely reduced. Expression of genes in the NOTCH signaling pathway involved in segmentation was significantly affected, and somite formation ceased after the production of about 15-20 somites. Defects seen in the mutants appear to result from a failure to produce sufficient paraxial mesoderm, rather than a failure of mesoderm precursors to migrate away from the primitive streak. Although the epiblast prematurely decreases in size, we did not detect evidence of a change in the proliferation rate of cells in the tail region or excessive apoptosis of epiblast or mesoderm cells. We propose that FGF4 and FGF8 are required to maintain a population of progenitor cells in the epiblast that generates mesoderm and contributes to the stem cell population that is incorporated in the tailbud and required for axial elongation of the mouse embryo after gastrulation.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




References
-
- Arenkiel BR, Gaufo GO, Capecchi MR. Hoxb1 neural crest preferentially form glia of the PNS. Dev Dyn. 2003;227:379–86. - PubMed
-
- Aulehla A, Wehrle C, Brand-Saberi B, Kemler R, Gossler A, Kanzler B, Herrmann BG. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell. 2003;4:395–406. - PubMed
-
- Beddington RS, Rashbass P, Wilson V. Brachyury--a gene affecting mouse gastrulation and early organogenesis. Dev Suppl. 1992:157–65. - PubMed
-
- Boulet AM, Capecchi MR. Targeted disruption of hoxc-4 causes esophageal defects and vertebral transformations. Dev Biol. 1996;177:232–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

