Improving the efficiency and relevance of evidence-based recommendations in the era of whole-genome sequencing: an EGAPP methods update
- PMID: 22955111
- PMCID: PMC3932295
- DOI: 10.1038/gim.2012.106
Improving the efficiency and relevance of evidence-based recommendations in the era of whole-genome sequencing: an EGAPP methods update
Abstract
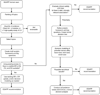
To provide an update on recent revisions to Evaluation of Genomic Applications in Practice and Prevention (EGAPP) methods designed to improve efficiency, and an assessment of the implications of whole genome sequencing for evidence-based recommendation development. Improvements to the EGAPP approach include automated searches for horizon scanning, a quantitative ranking process for topic prioritization, and the development of a staged evidence review and evaluation process. The staged process entails (i) triaging tests with minimal evidence of clinical validity, (ii) using and updating existing reviews, (iii) evaluating clinical validity prior to analytic validity or clinical utility, (iv) using decision modeling to assess potential clinical utility when direct evidence is not available. EGAPP experience to date suggests the following approaches will be critical for the development of evidence based recommendations in the whole genome sequencing era: (i) use of triage approaches and frameworks to improve efficiency, (ii) development of evidence thresholds that consider the value of further research, (iii) incorporation of patient preferences, and (iv) engagement of diverse stakeholders. The rapid advances in genomics present a significant challenge to traditional evidence based medicine, but also an opportunity for innovative approaches to recommendation development.
Conflict of interest statement
David Veenstra reports that he was a consultant for Medco, Novartis Molecular Diagnostics, and Genentech, and is supported by the following genomics-related research grants: P50HG003374, RC2CA148570, UO1GM092676, and UO1HG006507 from the National Institutes of Health and U18GD000005 from the Centers for Disease Control and Prevention. Stephen Pauker reports that a research study of his was supported by a fund from Novartis to Tufts Medical Center. Sean Tunis has no personal conflicts of interest to disclose. The Center for Medical Technology Policy receives funding from several sources, listed at
Figures
References
-
- Agency for Healthcare Research and Quality. Introduction to the methods guide for medical test reviews. [Accessed 21 May 21 2011];Methods Guide for Medical Test Reviews. 2010 http://www.effectivehealthcare.ahrq.gov/ehc/products/247/559/Paper01_%28....
-
- Haddow J, Palomaki G. ACCE: A Model Process for Evaluating Data on Emerging Genetic Tests. In: Khoury MLJ, Burke W, editors. Human Genome Epidemiology: A Scientific Foundation for Using Genetic Information to Improve Health and Prevent Disease. Oxford: Oxford University Press; 2003. pp. 217–233.
-
- Guirguis-Blake J, Calonge N, Miller T, Siu A, Teutsch S, Whitlock E. U.S. Preventive Services Task Force. Current processes of the U.S. Preventive Services Task Force: refining evidence-based recommendation development. Ann Intern Med. 2007;147:117–122. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical