Interleukin-10 inhibits lipopolysaccharide-induced tumor necrosis factor-α translation through a SHIP1-dependent pathway
- PMID: 22955274
- PMCID: PMC3488072
- DOI: 10.1074/jbc.M112.348599
Interleukin-10 inhibits lipopolysaccharide-induced tumor necrosis factor-α translation through a SHIP1-dependent pathway
Abstract
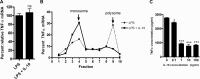
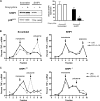
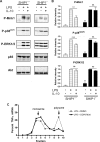
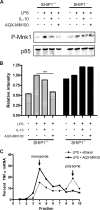
Production of the proinflammatory cytokine TNFα by activated macrophages is an important component of host defense. However, TNFα production must be tightly controlled to avoid pathological consequences. The anti-inflammatory cytokine IL-10 inhibits TNFα mRNA expression through activation of the STAT3 transcription factor pathway and subsequent expression of STAT3-dependent gene products. We hypothesized that IL-10 must also have more rapid mechanisms of action and show that IL-10 rapidly shifts existing TNFα mRNA from polyribosome-associated polysomes to monosomes. This translation suppression requires the presence of SHIP1 (SH2 domain-containing inositol 5'-phosphatase 1) and involves inhibition of Mnk1 (MAPK signal-integrating kinase 1). Furthermore, activating SHIP1 using a small-molecule agonist mimics the inhibitory effect of IL-10 on Mnk1 phosphorylation and TNFα translation. Our data support the existence of an alternative STAT3-independent pathway through SHIP1 for IL-10 to regulate TNFα translation during the anti-inflammatory response.
Figures
Similar articles
-
Src homology 2 domain-containing inositol-5-phosphatase 1 (SHIP1) negatively regulates TLR4-mediated LPS response primarily through a phosphatase activity- and PI-3K-independent mechanism.Blood. 2005 Jun 15;105(12):4685-92. doi: 10.1182/blood-2005-01-0191. Epub 2005 Feb 8. Blood. 2005. PMID: 15701712
-
Small-molecule agonists of SHIP1 inhibit the phosphoinositide 3-kinase pathway in hematopoietic cells.Blood. 2007 Sep 15;110(6):1942-9. doi: 10.1182/blood-2007-03-079699. Epub 2007 May 14. Blood. 2007. PMID: 17502453
-
Interleukin-10 inhibits lipopolysaccharide induced miR-155 precursor stability and maturation.PLoS One. 2013 Aug 12;8(8):e71336. doi: 10.1371/journal.pone.0071336. eCollection 2013. PLoS One. 2013. PMID: 23951138 Free PMC article.
-
The role of SHIP1 in macrophage programming and activation.Biochem Soc Trans. 2004 Nov;32(Pt 5):785-8. doi: 10.1042/BST0320785. Biochem Soc Trans. 2004. PMID: 15494015 Review.
-
Role of SHIP1 in bone biology.Ann N Y Acad Sci. 2013 Mar;1280:11-4. doi: 10.1111/nyas.12091. Ann N Y Acad Sci. 2013. PMID: 23551095 Free PMC article. Review.
Cited by
-
Interleukin-10 and Small Molecule SHIP1 Allosteric Regulators Trigger Anti-inflammatory Effects through SHIP1/STAT3 Complexes.iScience. 2020 Aug 21;23(8):101433. doi: 10.1016/j.isci.2020.101433. Epub 2020 Aug 2. iScience. 2020. PMID: 32823063 Free PMC article.
-
Regulator Versus Effector Paradigm: Interleukin-10 as Indicator of the Switching Response.Clin Rev Allergy Immunol. 2016 Feb;50(1):97-113. doi: 10.1007/s12016-015-8514-7. Clin Rev Allergy Immunol. 2016. PMID: 26450621 Review.
-
Genomic analysis of LPS-stimulated myeloid cells identifies a common pro-inflammatory response but divergent IL-10 anti-inflammatory responses.Sci Rep. 2015 Mar 13;5:9100. doi: 10.1038/srep09100. Sci Rep. 2015. PMID: 25765318 Free PMC article.
-
STAT3 signaling in immunity.Cytokine Growth Factor Rev. 2016 Oct;31:1-15. doi: 10.1016/j.cytogfr.2016.05.001. Epub 2016 May 9. Cytokine Growth Factor Rev. 2016. PMID: 27185365 Free PMC article. Review.
-
Hyporesponsiveness to the anti-inflammatory action of interleukin-10 in type 2 diabetes.Sci Rep. 2016 Feb 17;6:21244. doi: 10.1038/srep21244. Sci Rep. 2016. PMID: 26883847 Free PMC article.
References
-
- Chow J. C., Young D. W., Golenbock D. T., Christ W. J., Gusovsky F. (1999) Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274, 10689–10692 - PubMed
-
- Kruys V., Kemmer K., Shakhov A., Jongeneel V., Beutler B. (1992) Constitutive activity of the tumor necrosis factor promoter is canceled by the 3′-untranslated region in nonmacrophage cell lines; a trans-dominant factor overcomes this suppressive effect. Proc. Natl. Acad. Sci. U.S.A. 89, 673–677 - PMC - PubMed
-
- Buxadé M., Parra J. L., Rousseau S., Shpiro N., Marquez R., Morrice N., Bain J., Espel E., Proud C. G. (2005) The Mnks are novel components in the control of TNFα biosynthesis and phosphorylate and regulate hnRNP A1. Immunity 23, 177–189 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

