Nesfatin-1 as a novel cardiac peptide: identification, functional characterization, and protection against ischemia/reperfusion injury
- PMID: 22955491
- PMCID: PMC11113865
- DOI: 10.1007/s00018-012-1138-7
Nesfatin-1 as a novel cardiac peptide: identification, functional characterization, and protection against ischemia/reperfusion injury
Abstract
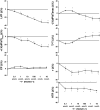
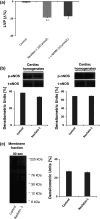
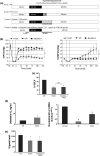
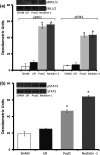
Nesfatin-1 is an anorexic nucleobindin-2 (NUCB2)-derived hypothalamic peptide. It controls feeding behavior, water intake, and glucose homeostasis. If intracerebrally administered, it induces hypertension, thus suggesting a role in central cardiovascular control. However, it is not known whether it is able to directly control heart performance. We aimed to verify the hypothesis that, as in the case of other hypothalamic satiety peptides, Nesfatin-1 acts as a peripheral cardiac modulator. By western blotting and QT-PCR, we identified the presence of both Nesfatin-1 protein and NUCB2 mRNA in rat cardiac extracts. On isolated and Langendorff-perfused rat heart preparations, we found that exogenous Nesfatin-1 depresses contractility and relaxation without affecting coronary motility. These effects did not involve Nitric oxide, but recruited the particulate guanylate cyclase (pGC) known as natriuretic peptide receptor A (NPR-A), protein kinase G (PKG) and extracellular signal-regulated kinases1/2 (ERK1/2). Co-immunoprecipitation and bioinformatic analyses supported an interaction between Nesfatin-1 and NPR-A. Lastly, we preliminarily observed, through post-conditioning experiments, that Nesfatin-1 protects against ischemia/reperfusion (I/R) injury by reducing infarct size, lactate dehydrogenase release, and postischemic contracture. This protection involves multiple prosurvival kinases such as PKCε, ERK1/2, signal transducer and activator of transcription 3, and mitochondrial K(ATP) channels. It also ameliorates contractility recovery. Our data indicate that: (1) the heart expresses Nesfatin-1, (2) Nesfatin-1 directly affects myocardial performance, possibly involving pGC-linked NPR-A, the pGC/PKG pathway, and ERK1/2, (3) the peptide protects the heart against I/R injury. Results pave the way to include Nesfatin-1 in the neuroendocrine modulators of the cardiac function, also encouraging the clarification of its clinical potential in the presence of nutrition-dependent physio-pathologic cardiovascular diseases.
Conflict of interest statement
None.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

