Mechanism for Hypocretin-mediated sleep-to-wake transitions
- PMID: 22955882
- PMCID: PMC3465396
- DOI: 10.1073/pnas.1202526109
Mechanism for Hypocretin-mediated sleep-to-wake transitions
Abstract
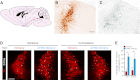
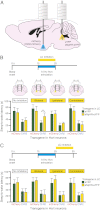
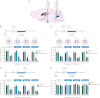
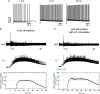
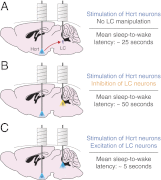
Current models of sleep/wake regulation posit that Hypocretin (Hcrt)-expressing neurons in the lateral hypothalamus promote and stabilize wakefulness by projecting to subcortical arousal centers. However, the critical downstream effectors of Hcrt neurons are unknown. Here we use optogenetic, pharmacological, and computational tools to investigate the functional connectivity between Hcrt neurons and downstream noradrenergic neurons in the locus coeruleus (LC) during nonrapid eye movement (NREM) sleep. We found that photoinhibiting LC neurons during Hcrt stimulation blocked Hcrt-mediated sleep-to-wake transitions. In contrast, when LC neurons were optically stimulated to increase membrane excitability, concomitant photostimulation of Hcrt neurons significantly increased the probability of sleep-to-wake transitions compared with Hcrt stimulation alone. We also built a conductance-based computational model of Hcrt-LC circuitry that recapitulates our behavioral results using LC neurons as the main effectors of Hcrt signaling. These results establish the Hcrt-LC connection as a critical integrator-effector circuit that regulates NREM sleep/wake behavior during the inactive period. This coupling of distinct neuronal systems can be generalized to other hypothalamic integrator nuclei with downstream effector/output populations in the brain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. - PubMed
-
- Pace-Schott EF, Hobson JA. The neurobiology of sleep: Genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. 2002;3:591–605. - PubMed
-
- Sakurai T, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. - PubMed
-
- Sutcliffe JG, de Lecea L. The hypocretins: Setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH83702/MH/NIMH NIH HHS/United States
- R01 MH083702/MH/NIMH NIH HHS/United States
- NS12151/NS/NINDS NIH HHS/United States
- T32 MH020016/MH/NIMH NIH HHS/United States
- MH87592/MH/NIMH NIH HHS/United States
- R01 MH087592/MH/NIMH NIH HHS/United States
- P01 NS012151/NS/NINDS NIH HHS/United States
- R01DC11422/DC/NIDCD NIH HHS/United States
- P50 NS012151/NS/NINDS NIH HHS/United States
- R01 NS006477/NS/NINDS NIH HHS/United States
- R01 DC011422/DC/NIDCD NIH HHS/United States
- R01 DA021880/DA/NIDA NIH HHS/United States
- F31MH83439/MH/NIMH NIH HHS/United States
- DA21880/DA/NIDA NIH HHS/United States
- R01 NS034774/NS/NINDS NIH HHS/United States
- F31 MH083439/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

