HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease
- PMID: 22955919
- PMCID: PMC3507140
- DOI: 10.1182/blood-2012-07-438408
HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease
Abstract
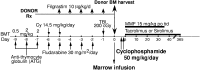
Allogeneic marrow transplantation can cure sickle cell disease; however, HLA-matched donors are difficult to find, and the toxicities of myeloablative conditioning are prohibitive for most adults with this disease. We developed a nonmyeloablative bone marrow transplantation platform using related, including HLA-haploidentical, donors for patients with sickle cell disease. The regimen consisted of antithymocyte globulin, fludarabine, cyclophosphamide, and total body irradiation, and graft-versus-host disease prophylaxis with posttransplantation high-dose cyclophosphamide, mycophenolate mofetil, and tacrolimus or sirolimus. After screening 19 patients, we transplanted 17, 14 from HLA-haploidentical and 3 from HLA-matched related donors. Eleven patients engrafted durably. With a median follow-up of 711 days (minimal follow up 224 days), 10 patients are asymptomatic, and 6 patients are off immunosupression. Only 1 patient developed skin-only acute graft-versus-host disease that resolved without any therapy; no mortality was seen. Nonmyeloablative conditioning with posttransplantation high-dose cyclophosphamide expands the donor pool, making marrow transplantation feasible for most patients with sickle cell disease, and is associated with a low risk of complications, even with haploidentical related donors. Graft failure, 43% in haploidentical pairs, remains a major obstacle but may be acceptable in a fraction of patients if the majority can be cured without serious toxicities.
Figures
Comment in
-
Haplo-BMT: cure or back to sickle cell?Blood. 2012 Nov 22;120(22):4276-7. doi: 10.1182/blood-2012-09-455832. Blood. 2012. PMID: 23175658 No abstract available.
References
-
- Lorey FW, Arnopp J, Cunningham GC. Distribution of hemoglobinopathy variants by ethnicity in a multiethnic state. Genet Epidemiol. 1996;13(5):501–512. - PubMed
-
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine. 2005;84(6):363–376. - PubMed
-
- Bernaudin F, Socié G, Kuentz M, et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007;110(7):2749–2756. - PubMed
-
- Walters MC, Patience M, Leisenring W, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335(6):369–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials