A novel cellular stress response characterised by a rapid reorganisation of membranes of the endoplasmic reticulum
- PMID: 22955944
- PMCID: PMC3504701
- DOI: 10.1038/cdd.2012.108
A novel cellular stress response characterised by a rapid reorganisation of membranes of the endoplasmic reticulum
Abstract
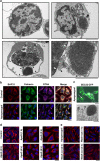
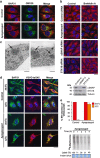
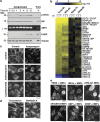
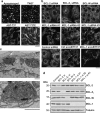
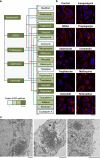
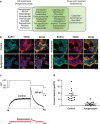
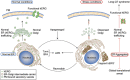
Canonical endoplasmic reticulum (ER) stress, which occurs in many physiological and disease processes, results in activation of the unfolded protein response (UPR). We now describe a new, evolutionarily conserved cellular stress response characterised by a striking, but reversible, reorganisation of ER membranes that occurs independently of the UPR, resulting in impaired ER transport and function. This reorganisation is characterised by a dramatic redistribution and clustering of ER membrane proteins. ER membrane aggregation is regulated, in part, by anti-apoptotic BCL-2 family members, particularly MCL-1. Using connectivity mapping, we report the widespread occurrence of this stress response by identifying several structurally diverse chemicals from different pharmacological classes, including antihistamines, antimalarials and antipsychotics, which induce ER membrane reorganisation. Furthermore, we demonstrate the potential of ER membrane aggregation to result in pathological consequences, such as the long-QT syndrome, a cardiac arrhythmic abnormality, arising because of a novel trafficking defect of the human ether-a-go-go-related channel protein from the ER to the plasma membrane. Thus, ER membrane reorganisation is a feature of a new cellular stress pathway, clearly distinct from the UPR, with important consequences affecting the normal functioning of the ER.
Figures
References
-
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. - PubMed
-
- Baumann O, Walz B. Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int Rev Cytol. 2001;205:149–214. - PubMed
-
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

