Cardiomyogenesis in the aging and failing human heart
- PMID: 22955965
- PMCID: PMC3477474
- DOI: 10.1161/CIRCULATIONAHA.112.118380
Cardiomyogenesis in the aging and failing human heart
Retraction in
-
Notice of retraction.Circulation. 2014 Apr 22;129(16):e466. doi: 10.1161/CIR.0000000000000049. Epub 2014 Apr 8. Circulation. 2014. PMID: 24714642 Free PMC article. No abstract available.
Abstract
Background: Two opposite views of cardiac growth are currently held; one views the heart as a static organ characterized by a large number of cardiomyocytes that are present at birth and live as long as the organism, and the other views the heart a highly plastic organ in which the myocyte compartment is restored several times during the course of life.
Methods and results: The average age of cardiomyocytes, vascular endothelial cells (ECs), and fibroblasts and their turnover rates were measured by retrospective (14)C birth dating of cells in 19 normal hearts 2 to 78 years of age and in 17 explanted failing hearts 22 to 70 years of age. We report that the human heart is characterized by a significant turnover of ventricular myocytes, ECs, and fibroblasts, physiologically and pathologically. Myocyte, EC, and fibroblast renewal is very high shortly after birth, decreases during postnatal maturation, remains relatively constant in the adult organ, and increases dramatically with age. From 20 to 78 years of age, the adult human heart entirely replaces its myocyte, EC, and fibroblast compartment ≈8, ≈6, and ≈8 times, respectively. Myocyte, EC, and fibroblast regeneration is further enhanced with chronic heart failure.
Conclusions: The human heart is a highly dynamic organ that retains a remarkable degree of plasticity throughout life and in the presence of chronic heart failure. However, the ability to regenerate cardiomyocytes, vascular ECs, and fibroblasts cannot prevent the manifestations of myocardial aging or oppose the negative effects of ischemic and idiopathic dilated cardiomyopathy.
Figures
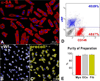




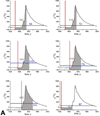

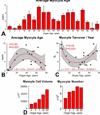

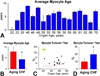






Comment in
-
On the fabric of the human body.Circulation. 2012 Oct 9;126(15):1812-4. doi: 10.1161/CIRCULATIONAHA.112.136127. Epub 2012 Sep 6. Circulation. 2012. PMID: 22955964 No abstract available.
References
-
- Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. - PubMed
-
- Kajstura J, Gurusamy N, Ogórek B, Goichberg P, Clavo-Rondon C, Hosoda T, D'Amario D, Bardelli S, Beltrami AP, Cesselli D, Bussani R, del Monte F, Quaini F, Rota M, Beltrami CA, Buchholz BA, Leri A, Anversa P. Myocyte turnover in the aging human heart. Circ Res. 2010;107:1374–1386. - PubMed
-
- Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AG017042/AG/NIA NIH HHS/United States
- R01 HL111183/HL/NHLBI NIH HHS/United States
- P41 RR013461/RR/NCRR NIH HHS/United States
- R01 HL075480/HL/NHLBI NIH HHS/United States
- R01 HL114346/HL/NHLBI NIH HHS/United States
- P01 HL092868/HL/NHLBI NIH HHS/United States
- R01 HL102897/HL/NHLBI NIH HHS/United States
- RR13461/RR/NCRR NIH HHS/United States
- R01 HL091021/HL/NHLBI NIH HHS/United States
- R01 HL039902/HL/NHLBI NIH HHS/United States
- R01 HL105532/HL/NHLBI NIH HHS/United States
- R01 AG037495/AG/NIA NIH HHS/United States
- R01 AG026107/AG/NIA NIH HHS/United States
- R01 HL065577/HL/NHLBI NIH HHS/United States
- R01 HL065573/HL/NHLBI NIH HHS/United States
- R37 HL081737/HL/NHLBI NIH HHS/United States
- R01 AG037490/AG/NIA NIH HHS/United States
- P01 HL078825/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

