Intracellular chloride channel protein CLIC1 regulates macrophage function through modulation of phagosomal acidification
- PMID: 22956539
- PMCID: PMC3561857
- DOI: 10.1242/jcs.110072
Intracellular chloride channel protein CLIC1 regulates macrophage function through modulation of phagosomal acidification
Abstract
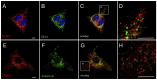
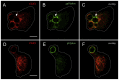
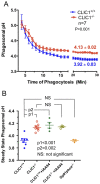
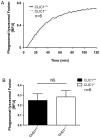
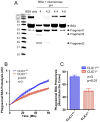
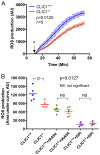
Intracellular chloride channel protein 1 (CLIC1) is a 241 amino acid protein of the glutathione S transferase fold family with redox- and pH-dependent membrane association and chloride ion channel activity. Whilst CLIC proteins are evolutionarily conserved in Metazoa, indicating an important role, little is known about their biology. CLIC1 was first cloned on the basis of increased expression in activated macrophages. We therefore examined its subcellular localisation in murine peritoneal macrophages by immunofluorescence confocal microscopy. In resting cells, CLIC1 is observed in punctate cytoplasmic structures that do not colocalise with markers for endosomes or secretory vesicles. However, when these macrophages phagocytose serum-opsonised zymosan, CLIC1 translocates onto the phagosomal membrane. Macrophages from CLIC1(-/-) mice display a defect in phagosome acidification as determined by imaging live cells phagocytosing zymosan tagged with the pH-sensitive fluorophore Oregon Green. This altered phagosomal acidification was not accompanied by a detectable impairment in phagosomal-lysosomal fusion. However, consistent with a defect in acidification, CLIC1(-/-) macrophages also displayed impaired phagosomal proteolytic capacity and reduced reactive oxygen species production. Further, CLIC1(-/-) mice were protected from development of serum transfer induced K/BxN arthritis. These data all point to an important role for CLIC1 in regulating macrophage function through its ion channel activity and suggest it is a suitable target for the development of anti-inflammatory drugs.
Figures



References
-
- Barriere H., Bagdany M., Bossard F., Okiyoneda T., Wojewodka G., Gruenert D., Radzioch D., Lukacs G. L. (2009). Revisiting the role of cystic fibrosis transmembrane conductance regulator and counterion permeability in the pH regulation of endocytic organelles. Mol. Biol. Cell 20, 3125–3141 10.1091/mbc.E09-01-0061 - DOI - PMC - PubMed
-
- Bradford E. M., Miller M. L., Prasad V., Nieman M. L., Gawenis L. R., Berryman M., Lorenz J. N., Tso P., Shull G. E. (2010) CLIC5 mutant mice are resistant to diet-induced obesity and exhibit gastric hemorrhaging and increased susceptibility to torpor. Am. J. Physiol. 298, R1531–1542 10.1152/ajpregu.00849.2009 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

