Existence of the canonical Wnt signaling pathway in the human trabecular meshwork
- PMID: 22956608
- PMCID: PMC4607241
- DOI: 10.1167/iovs.12-9664
Existence of the canonical Wnt signaling pathway in the human trabecular meshwork
Abstract
Purpose: We previously discovered elevated levels of secreted frizzled-related protein 1 (sFRP1), the Wnt signaling pathway inhibitor, in the glaucomatous trabecular meshwork (GTM), and found that key canonical Wnt signaling pathway genes are expressed in the trabecular meshwork (TM). The purpose of our study was to determine whether a functional canonical Wnt signaling pathway exists in the human TM (HTM).
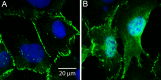
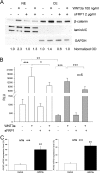
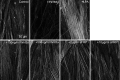
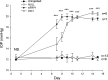
Methods: Western immunoblotting and/or immunofluorescent microscopy were used to study β-catenin translocation as well as the actin cytoskeleton in transformed and primary HTM cells. A TCF/LEF luciferase assay was used to study functional canonical Wnt signaling, which was confirmed further by WNT3a-induced expression of a pathway target gene, AXIN2, via quantitative PCR. Intravitreal injection of an Ad5 adenovirus expressing Dickkopf-related protein-1 (DKK1) was used to study the in vivo effect of canonical Wnt signaling on IOP in mice.
Results: WNT3a induced β-catenin translocation in the HTM, which was blocked by co-treatment with sFRP1. Similarly, WNT3a enhanced luciferase levels in TCF/LEF luciferase assays, which also were blocked by sFRP1. Furthermore, AXIN2 expression was elevated significantly by WNT3a. However, neither WNT3a nor sFRP1 affected actin cytoskeleton organization, which theoretically could be regulated by noncanonical Wnt signaling in HTM cells. Exogenous DKK1, a specific inhibitor for the canonical Wnt signaling pathway, or sFRP1 elevated mouse IOP to equivalent levels.
Conclusions: There is a canonical Wnt signaling pathway in the TM, and this canonical Wnt pathway, but not the noncanonical Wnt signaling pathway, regulates IOP.
Conflict of interest statement
Disclosure:
Figures


Comment in
-
Existence of the canonical Wnt signaling pathway in the human trabecular meshwork.Invest Ophthalmol Vis Sci. 2012 Oct 9;53(11):6972. doi: 10.1167/iovs.12-10985. Print 2012 Oct. Invest Ophthalmol Vis Sci. 2012. PMID: 23047718 No abstract available.
References
-
- The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000; 130: 429– 440. - PubMed
-
- Heijl A, Leske MC, Bengtsson B, Hyman L, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002; 120: 1268– 1279. - PubMed
-
- Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120: 701– 713 ; discussion 829–830. - PubMed
-
- Lichter PR. Impact of intraocular pressure reduction on glaucoma progression. JAMA. 2002; 288: 2607– 2608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

