Effects of parabens on adipocyte differentiation
- PMID: 22956630
- PMCID: PMC3621350
- DOI: 10.1093/toxsci/kfs262
Effects of parabens on adipocyte differentiation
Abstract
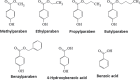
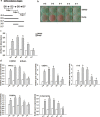
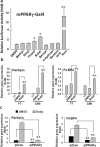
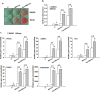
Parabens are a group of alkyl esters of p-hydroxybenzoic acid that include methylparaben, ethylparaben, propylparaben, butylparaben, and benzylparaben. Paraben esters and their salts are widely used as preservatives in cosmetics, toiletries, food, and pharmaceuticals. Humans are exposed to parabens through the use of such products from dermal contact, ingestion, and inhalation. However, research on the effects of parabens on health is limited, and the effects of parabens on adipogenesis have not been systematically studied. Here, we report that (1) parabens promote adipogenesis (or adipocyte differentiation) in murine 3T3-L1 cells, as revealed by adipocyte morphology, lipid accumulation, and mRNA expression of adipocyte-specific markers; (2) the adipogenic potency of parabens is increased with increasing length of the linear alkyl chain in the following potency ranking order: methyl- < ethyl- < propyl- < butylparaben. The extension of the linear alkyl chain with an aromatic ring in benzylparaben further augments the adipogenic ability, whereas 4-hydroxybenzoic acid, the common metabolite of all parabens, and the structurally related benzoic acid (without the OH group) are inactive in promoting 3T3-L1 adipocyte differentiation; (3) parabens activate glucocorticoid receptor and/or peroxisome proliferator-activated receptor γ in 3T3-L1 preadipocytes; however, no direct binding to, or modulation of, the ligand binding domain of the glucocorticoid receptor by parabens was detected by glucocorticoid receptor competitor assays; and lastly, (4) parabens, butyl- and benzylparaben in particular, also promote adipose conversion of human adipose-derived multipotent stromal cells. Our results suggest that parabens may contribute to obesity epidemic, and the role of parabens in adipogenesis in vivo needs to be examined further.
Figures




References
-
- Arimura N., Horiba T., Imagawa M., Shimizu M., Sato R. (2004). The peroxisome proliferator-activated receptor gamma regulates expression of the perilipin gene in adipocytes. J. Biol. Chem. 279, 10070–10076. - PubMed
-
- Bando H., Mohri S., Yamashita F., Takakura Y., Hashida M. (1997). Effects of skin metabolism on percutaneous penetration of lipophilic drugs. J. Pharm. Sci. 86, 759–761. - PubMed
-
- Boberg J., Metzdorff S., Wortziger R., Axelstad M., Brokken L., Vinggaard A. M., Dalgaard M., Nellemann C. (2008). Impact of diisobutyl phthal ate and other PPAR agonists on steroidogenesis and plasma insulin and leptin levels in fetal rats. Toxicology 250, 75–81. - PubMed
-
- Boberg J., Taxvig C., Christiansen S., Hass U. (2010). Possible endocrine disrupting effects of parabens and their metabolites. Reprod. Toxicol. 30, 301–312. - PubMed
-
- Byford J. R., Shaw L. E., Drew M. G., Pope G. S., Sauer M. J., Darbre P. D. (2002). Oestrogenic activity of parabens in MCF7 human breast cancer cells. J. Steroid Biochem. Mol. Biol. 80, 49–60. - PubMed

