The Hog1 SAPK controls the Rtg1/Rtg3 transcriptional complex activity by multiple regulatory mechanisms
- PMID: 22956768
- PMCID: PMC3484105
- DOI: 10.1091/mbc.E12-04-0289
The Hog1 SAPK controls the Rtg1/Rtg3 transcriptional complex activity by multiple regulatory mechanisms
Abstract
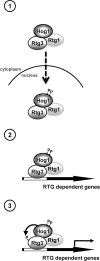
Cells modulate expression of nuclear genes in response to alterations in mitochondrial function, a response termed retrograde (RTG) regulation. In budding yeast, the RTG pathway relies on Rtg1 and Rtg3 basic helix-loop-helix leucine Zipper transcription factors. Exposure of yeast to external hyperosmolarity activates the Hog1 stress-activated protein kinase (SAPK), which is a key player in the regulation of gene expression upon stress. Several transcription factors, including Sko1, Hot1, the redundant Msn2 and Msn4, and Smp1, have been shown to be directly controlled by the Hog1 SAPK. The mechanisms by which Hog1 regulates their activity differ from one to another. In this paper, we show that Rtg1 and Rtg3 transcription factors are new targets of the Hog1 SAPK. In response to osmostress, RTG-dependent genes are induced in a Hog1-dependent manner, and Hog1 is required for Rtg1/3 complex nuclear accumulation. In addition, Hog1 activity regulates Rtg1/3 binding to chromatin and transcriptional activity. Therefore Hog1 modulates Rtg1/3 complex activity by multiple mechanisms in response to stress. Overall our data suggest that Hog1, through activation of the RTG pathway, contributes to ensure mitochondrial function as part of the Hog1-mediated osmoadaptive response.
Figures


Similar articles
-
Osmostress-induced transcription by Hot1 depends on a Hog1-mediated recruitment of the RNA Pol II.EMBO J. 2003 May 15;22(10):2433-42. doi: 10.1093/emboj/cdg243. EMBO J. 2003. PMID: 12743037 Free PMC article.
-
Dissection of the elements of osmotic stress response transcription factor Hot1 involved in the interaction with MAPK Hog1 and in the activation of transcription.Biochim Biophys Acta. 2013 Oct;1829(10):1111-25. doi: 10.1016/j.bbagrm.2013.07.009. Epub 2013 Aug 2. Biochim Biophys Acta. 2013. PMID: 23916462
-
The bidirectional cytomegalovirus immediate/early promoter is regulated by Hog1 and the stress transcription factors Sko1 and Hot1 in yeast.Mol Genet Genomics. 2010 May;283(5):511-8. doi: 10.1007/s00438-010-0537-4. Epub 2010 Apr 4. Mol Genet Genomics. 2010. PMID: 20364387
-
Multilayered control of gene expression by stress-activated protein kinases.EMBO J. 2010 Jan 6;29(1):4-13. doi: 10.1038/emboj.2009.346. Epub 2009 Nov 26. EMBO J. 2010. PMID: 19942851 Free PMC article. Review.
-
Osmostress-induced gene expression--a model to understand how stress-activated protein kinases (SAPKs) regulate transcription.FEBS J. 2015 Sep;282(17):3275-85. doi: 10.1111/febs.13323. Epub 2015 Jun 10. FEBS J. 2015. PMID: 25996081 Free PMC article. Review.
Cited by
-
The SrkA Kinase Is Part of the SakA Mitogen-Activated Protein Kinase Interactome and Regulates Stress Responses and Development in Aspergillus nidulans.Eukaryot Cell. 2015 May;14(5):495-510. doi: 10.1128/EC.00277-14. Epub 2015 Mar 27. Eukaryot Cell. 2015. PMID: 25820520 Free PMC article.
-
Role of spt23 in Saccharomyces cerevisiae thermal tolerance.Appl Microbiol Biotechnol. 2022 May;106(9-10):3691-3705. doi: 10.1007/s00253-022-11920-3. Epub 2022 Apr 27. Appl Microbiol Biotechnol. 2022. PMID: 35476152 Free PMC article.
-
Differential regulation of mitochondrial pyruvate carrier genes modulates respiratory capacity and stress tolerance in yeast.PLoS One. 2013 Nov 14;8(11):e79405. doi: 10.1371/journal.pone.0079405. eCollection 2013. PLoS One. 2013. PMID: 24244496 Free PMC article.
-
A Matlab-based application for quantification of yeast cell growth on solid media.J R Soc Interface. 2024 Mar;21(212):20230695. doi: 10.1098/rsif.2023.0695. Epub 2024 Mar 20. J R Soc Interface. 2024. PMID: 38503339 Free PMC article.
-
Coordinated gene regulation in the initial phase of salt stress adaptation.J Biol Chem. 2015 Apr 17;290(16):10163-75. doi: 10.1074/jbc.M115.637264. Epub 2015 Mar 5. J Biol Chem. 2015. PMID: 25745106 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

