Repeated stress dysregulates κ-opioid receptor signaling in the dorsal raphe through a p38α MAPK-dependent mechanism
- PMID: 22956823
- PMCID: PMC3477582
- DOI: 10.1523/JNEUROSCI.2053-12.2012
Repeated stress dysregulates κ-opioid receptor signaling in the dorsal raphe through a p38α MAPK-dependent mechanism
Abstract
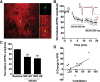
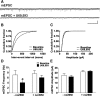
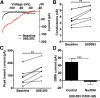
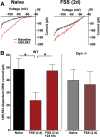
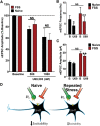
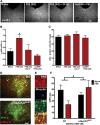
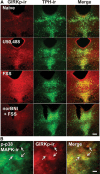
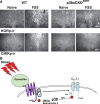
Repeated stress releases dynorphins and causes subsequent activation of κ-opioid receptors (KORs) in limbic brain regions. The serotonergic dorsal raphe nucleus (DRN) has previously been found to be an important site of action for the dysphoric effects of dynorphin-κ-opioid receptor system activation during stress-evoked behaviors, and KOR-induced activation of p38α mitogen-activated protein kinase (MAPK) in serotonergic neurons was found to be a critical mediator of the aversive properties of stress. Yet, how dynorphins and KORs functionally regulate the excitability of serotonergic DRN neurons both in adaptive and pathological stress states is poorly understood. Here we report that acute KOR activation by the selective agonist U69,593 [(+)-(5α,7α,8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]benzeneacetamide] inhibits serotonergic neuronal excitability within the DRN through both presynaptic inhibition of excitatory synaptic transmission and postsynaptic activation of G-protein-gated inwardly rectifying potassium channels (GIRKs) electrophysiologically recorded in brain slices. C57BL/6 mice subjected to repeated swim, stress sessions had significantly reduced KOR-mediated GIRK currents recorded in serotonergic neurons in DRN postsynaptically, without significantly affecting presynaptic KOR-mediated regulation of excitatory transmission. This effect was blocked by genetic excision of p38α MAPK selectively from serotonergic neurons. An increase in phospho-immunoreactivity suggests that this functional dysregulation may be a consequence of tyrosine phosphorylation of GIRK (K(IR)3.1) channels. These data elucidate a mechanism for stress-induced dysregulation of the excitability of neurons in the DRN and identify a functional target of stress-induced p38α MAPK activation that may underlie some of the negative effects of pathological stress exposure.
Figures


Similar articles
-
Kappa Opioid Receptor-Induced Aversion Requires p38 MAPK Activation in VTA Dopamine Neurons.J Neurosci. 2015 Sep 16;35(37):12917-31. doi: 10.1523/JNEUROSCI.2444-15.2015. J Neurosci. 2015. PMID: 26377476 Free PMC article.
-
Stress produces aversion and potentiates cocaine reward by releasing endogenous dynorphins in the ventral striatum to locally stimulate serotonin reuptake.J Neurosci. 2012 Dec 5;32(49):17582-96. doi: 10.1523/JNEUROSCI.3220-12.2012. J Neurosci. 2012. PMID: 23223282 Free PMC article.
-
Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking.Proc Natl Acad Sci U S A. 2009 Nov 10;106(45):19168-73. doi: 10.1073/pnas.0910705106. Epub 2009 Oct 28. Proc Natl Acad Sci U S A. 2009. PMID: 19864633 Free PMC article.
-
Kinase cascades and ligand-directed signaling at the kappa opioid receptor.Psychopharmacology (Berl). 2010 Jun;210(2):137-47. doi: 10.1007/s00213-010-1806-y. Epub 2010 Apr 17. Psychopharmacology (Berl). 2010. PMID: 20401607 Free PMC article. Review.
-
Dynorphin, stress, and depression.Brain Res. 2010 Feb 16;1314:56-73. doi: 10.1016/j.brainres.2009.09.074. Epub 2009 Sep 24. Brain Res. 2010. PMID: 19782055 Free PMC article. Review.
Cited by
-
The kappa opioid receptor: from addiction to depression, and back.Front Psychiatry. 2014 Dec 8;5:170. doi: 10.3389/fpsyt.2014.00170. eCollection 2014. Front Psychiatry. 2014. PMID: 25538632 Free PMC article. Review.
-
Functions of p38 MAP Kinases in the Central Nervous System.Front Mol Neurosci. 2020 Sep 8;13:570586. doi: 10.3389/fnmol.2020.570586. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33013322 Free PMC article. Review.
-
Stress alters social behavior and sensitivity to pharmacological activation of kappa opioid receptors in an age-specific manner in Sprague Dawley rats.Neurobiol Stress. 2018 Sep 11;9:124-132. doi: 10.1016/j.ynstr.2018.09.003. eCollection 2018 Nov. Neurobiol Stress. 2018. PMID: 30450378 Free PMC article.
-
Key differences in regulation of opioid receptors localized to presynaptic terminals compared to somas: Relevance for novel therapeutics.Neuropharmacology. 2023 Mar 15;226:109408. doi: 10.1016/j.neuropharm.2022.109408. Epub 2022 Dec 28. Neuropharmacology. 2023. PMID: 36584882 Free PMC article. Review.
-
Age as a factor in stress and alcohol interactions: A critical role for the kappa opioid system.Alcohol. 2018 Nov;72:9-18. doi: 10.1016/j.alcohol.2017.10.002. Epub 2017 Oct 12. Alcohol. 2018. PMID: 30322483 Free PMC article. Review.
References
-
- Adell A, Casanovas JM, Artigas F. Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology. 1997;36:735–741. - PubMed
-
- Azmitia EC, Gannon PJ. The primate serotonergic system: a review of human and animal studies and a report on Macaca fascicularis. Adv Neurol. 1986;43:407–468. - PubMed
-
- Bale TL. Stress sensitivity and the development of affective disorders. Horm Behav. 2006;50:529–533. - PubMed
-
- Bausch SB, Esteb TM, Terman GW, Chavkin C. Administered and endogenously released kappa opioids decrease pilocarpine-induced seizures and seizure-induced histopathology. J Pharmacol Exp Ther. 1998;284:1147–1155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
