Cholinergic coordination of presynaptic and postsynaptic activity induces timing-dependent hippocampal synaptic plasticity
- PMID: 22956824
- PMCID: PMC3474164
- DOI: 10.1523/JNEUROSCI.2129-12.2012
Cholinergic coordination of presynaptic and postsynaptic activity induces timing-dependent hippocampal synaptic plasticity
Abstract
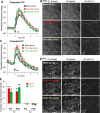
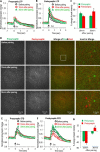
Correlated presynaptic and postsynaptic activity is the key factor in inducing Hebbian plasticity and memory. However, little is known about the physiological events that could mediate such coordination. Correlated cholinergic input induces spike timing-dependent plasticity-like hippocampal synaptic plasticity. Cholinergic receptors are localized to both presynaptic and postsynaptic glutamatergic sites and thus have the potential to coordinate presynaptic and postsynaptic activity to induce plasticity. By directly monitoring presynaptic and postsynaptic activities with genetically encoded calcium indicators in mouse septohippocampal cocultures, we found interactive but independent presynaptic and postsynaptic modulations in the cholinergic-dependent synaptic plasticity. Neither presynaptic nor postsynaptic modulation alone is sufficient, but instead a coordinated modulation at both sites is required to induce the plasticity. Therefore, we propose that correlated cholinergic input can coordinate presynaptic and postsynaptic activities to induce timing-dependent synaptic plasticity, providing a novel mechanism by which neuromodulators precisely modulate network activity and plasticity with high efficiency and temporal precision.
Figures





References
-
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nat Rev Neurosci. 2000;1:11–20. - PubMed
-
- Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature. 1997;387:278–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
