Optogenetic probing of fast glutamatergic transmission from hypocretin/orexin to histamine neurons in situ
- PMID: 22956835
- PMCID: PMC6621251
- DOI: 10.1523/JNEUROSCI.0706-12.2012
Optogenetic probing of fast glutamatergic transmission from hypocretin/orexin to histamine neurons in situ
Abstract
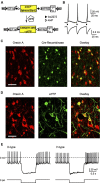
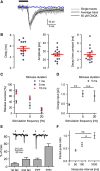
Hypothalamic hypocretin/orexin (hcrt/orx) neurons coordinate sleep-wake cycles, reward seeking, and body energy balance. Neurochemical data suggest that hcrt/orx cells contain several transmitters, but what hcrt/orx cells release onto their projection targets is unknown. A major pathway by which hcrt/orx neurons are thought to promote arousal is through projections to tuberomammillary histamine (HA) neurons. To study the impact of the electrical activity in hcrt/orx cells on HA neurons, we genetically targeted the light-activated excitatory ion channel channelrhodopsin-2 (ChR2) to the plasma membrane of hcrt/orx cells, and performed patch-clamp recordings from HA cells in acute mouse brain slices. Stimulation of ChR2-containing fibers with millisecond flashes of blue light produced fast postsynaptic currents in HA neurons, with a high connection probability (≈60% of HA cells were connected to ≈40% of hcrt/orx cells expressing ChR2). These inputs depended on tetrodotoxin-sensitive action potentials, had kinetics typical of glutamatergic responses mediated by AMPA receptors, were blocked by the AMPA receptor blocker CNQX, and displayed multiple forms of short-term plasticity (depression in ≈70% trials, facilitation in ≈30% trials, both often in the same cell). Furthermore, stimulation of hcrt/orx axons at physiological frequencies rapidly and reversibly increased action potential firing in HA cells, an effect that was abolished by blockade of AMPA receptors. These results provide the first functional evidence that hcrt/orx neurons are capable of fast glutamatergic control of their projection targets, and suggest that variations in electrical activity of hcrt/orx axons can induce rapid changes in long-range signals generated by HA neurons.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
