Reward cues in space: commonalities and differences in neural coding by hippocampal and ventral striatal ensembles
- PMID: 22956836
- PMCID: PMC3492752
- DOI: 10.1523/JNEUROSCI.0593-12.2012
Reward cues in space: commonalities and differences in neural coding by hippocampal and ventral striatal ensembles
Abstract
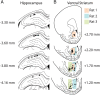
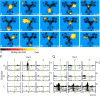
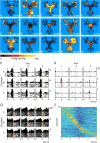
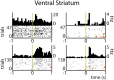
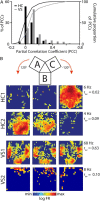
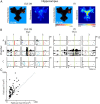
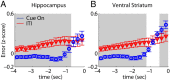
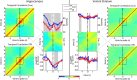
Forming place-reward associations critically depends on the integrity of the hippocampal-ventral striatal system. The ventral striatum (VS) receives a strong hippocampal input conveying spatial-contextual information, but it is unclear how this structure integrates this information to invigorate reward-directed behavior. Neuronal ensembles in rat hippocampus (HC) and VS were simultaneously recorded during a conditioning task in which navigation depended on path integration. In contrast to HC, ventral striatal neurons showed low spatial selectivity, but rather coded behavioral task phases toward reaching goal sites. Outcome-predicting cues induced a remapping of firing patterns in the HC, consistent with its role in episodic memory. VS remapped in conjunction with the HC, indicating that remapping can take place in multiple brain regions engaged in the same task. Subsets of ventral striatal neurons showed a "flip" from high activity when cue lights were illuminated to low activity in intertrial intervals, or vice versa. The cues induced an increase in spatial information transmission and sparsity in both structures. These effects were paralleled by an enhanced temporal specificity of ensemble coding and a more accurate reconstruction of the animal's position from population firing patterns. Altogether, the results reveal strong differences in spatial processing between hippocampal area CA1 and VS, but indicate similarities in how discrete cues impact on this processing.
Figures


Similar articles
-
Reward Expectancy Strengthens CA1 Theta and Beta Band Synchronization and Hippocampal-Ventral Striatal Coupling.J Neurosci. 2016 Oct 12;36(41):10598-10610. doi: 10.1523/JNEUROSCI.0682-16.2016. J Neurosci. 2016. PMID: 27733611 Free PMC article.
-
Hippocampus leads ventral striatum in replay of place-reward information.PLoS Biol. 2009 Aug;7(8):e1000173. doi: 10.1371/journal.pbio.1000173. Epub 2009 Aug 18. PLoS Biol. 2009. PMID: 19688032 Free PMC article.
-
Striatal versus hippocampal representations during win-stay maze performance.J Neurophysiol. 2009 Mar;101(3):1575-87. doi: 10.1152/jn.91106.2008. Epub 2009 Jan 14. J Neurophysiol. 2009. PMID: 19144741 Free PMC article.
-
Reactivation in ventral striatum during hippocampal ripples: evidence for the binding of reward and spatial memories?J Neurosci. 2008 Oct 1;28(40):9895-7. doi: 10.1523/JNEUROSCI.3778-08.2008. J Neurosci. 2008. PMID: 18829947 Free PMC article. Review. No abstract available.
-
Parallel processing across neural systems: implications for a multiple memory system hypothesis.Neurobiol Learn Mem. 2004 Nov;82(3):278-98. doi: 10.1016/j.nlm.2004.07.007. Neurobiol Learn Mem. 2004. PMID: 15464410 Review.
Cited by
-
Transient DREADD Manipulation of the Dorsal Dentate Gyrus in Rats Impairs Initial Learning of Place-Outcome Associations.Hippocampus. 2025 May;35(3):e70014. doi: 10.1002/hipo.70014. Hippocampus. 2025. PMID: 40326013 Free PMC article.
-
Task-dependent mixed selectivity in the subiculum.Cell Rep. 2021 May 25;35(8):109175. doi: 10.1016/j.celrep.2021.109175. Cell Rep. 2021. PMID: 34038726 Free PMC article.
-
Reassessing wanting and liking in the study of mesolimbic influence on food intake.Am J Physiol Regul Integr Comp Physiol. 2016 Nov 1;311(5):R811-R840. doi: 10.1152/ajpregu.00234.2016. Epub 2016 Aug 17. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 27534877 Free PMC article. Review.
-
Coherent mapping of position and head direction across auditory and visual cortex.Cereb Cortex. 2023 Jun 8;33(12):7369-7385. doi: 10.1093/cercor/bhad045. Cereb Cortex. 2023. PMID: 36967108 Free PMC article.
-
Memories of Opiate Withdrawal Emotional States Correlate with Specific Gamma Oscillations in the Nucleus Accumbens.Neuropsychopharmacology. 2017 Apr;42(5):1157-1168. doi: 10.1038/npp.2016.272. Epub 2016 Dec 6. Neuropsychopharmacology. 2017. PMID: 27922595 Free PMC article.
References
-
- Aggleton JP, Petrides M, Iversen SD. Differential effects of amygdaloid lesions on conditioned taste aversion learning by rats. Physiol Behav. 1981;27:397–400. - PubMed
-
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. - PubMed
-
- Annett LE, McGregor A, Robbins TW. The effects of ibotenic acid lesions of the nucleus accumbens on spatial learning and extinction in the rat. Behav Brain Res. 1989;31:231–242. - PubMed
-
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
