Predicting perceptual decision biases from early brain activity
- PMID: 22956839
- PMCID: PMC6621270
- DOI: 10.1523/JNEUROSCI.1708-12.2012
Predicting perceptual decision biases from early brain activity
Abstract
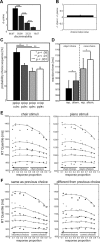
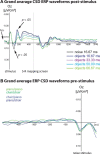
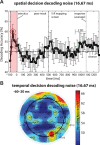
Perceptual decision making is believed to be driven by the accumulation of sensory evidence following stimulus encoding. More controversially, some studies report that neural activity preceding the stimulus also affects the decision process. We used a multivariate pattern classification approach for the analysis of the human electroencephalogram (EEG) to decode choice outcomes in a perceptual decision task from spatially and temporally distributed patterns of brain signals. When stimuli provided discriminative information, choice outcomes were predicted by neural activity following stimulus encoding; when stimuli provided no discriminative information, choice outcomes were predicted by neural activity preceding the stimulus. Moreover, in the absence of discriminative information, the recent choice history primed the choices on subsequent trials. A diffusion model fitted to the choice probabilities and response time distributions showed that the starting point of the evidence accumulation process was shifted toward the previous choice, consistent with the hypothesis that choice priming biases the accumulation process toward a decision boundary. This bias is reflected in prestimulus brain activity, which, in turn, becomes predictive of future decisions. Our results provide a model of how non-stimulus-driven decision making in humans could be accomplished on a neural level.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
