Myelin gene regulatory factor is required for maintenance of myelin and mature oligodendrocyte identity in the adult CNS
- PMID: 22956843
- PMCID: PMC3752083
- DOI: 10.1523/JNEUROSCI.1069-12.2012
Myelin gene regulatory factor is required for maintenance of myelin and mature oligodendrocyte identity in the adult CNS
Abstract
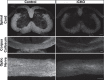
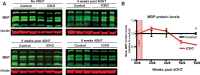
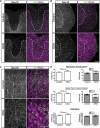
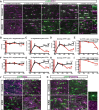
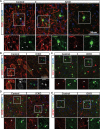
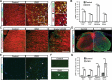
Although the transcription factors required for the generation of oligodendrocytes and CNS myelination during development have been relatively well established, it is not known whether continued expression of the same factors is required for the maintenance of myelin in the adult. Here, we use an inducible conditional knock-out strategy to investigate whether continued oligodendrocyte expression of the recently identified transcription factor myelin gene regulatory factor (MRF) is required to maintain the integrity of myelin in the adult CNS. Genetic ablation of MRF in mature oligodendrocytes within the adult CNS resulted in a delayed but severe CNS demyelination, with clinical symptoms beginning at 5 weeks and peaking at 8 weeks after ablation of MRF. This demyelination was accompanied by microglial/macrophage infiltration and axonal damage. Transcripts for myelin genes, such as proteolipid protein, MAG, MBP, and myelin oligodendrocyte glycoprotein, were rapidly downregulated after ablation of MRF, indicating an ongoing requirement for MRF in the expression of these genes. Subsequently, a proportion of the recombined oligodendrocytes undergo apoptosis over a period of weeks. Surviving oligodendrocytes gradually lose the expression of mature markers such as CC1 antigen and their association with myelin, without reexpressing oligodendrocyte progenitor markers or reentering the cell cycle. These results demonstrate that ongoing expression of MRF within the adult CNS is critical to maintain mature oligodendrocyte identity and the integrity of CNS myelin.
Figures




References
-
- Aggarwal S, Yurlova L, Simons M. Central nervous system myelin: structure, synthesis and assembly. Trends Cell Biol. 2011;21:585–593. - PubMed
-
- Agrawal D, Hawk R, Avila RL, Inouye H, Kirschner DA. Internodal myelination during development quantitated using X-ray diffraction. J Struct Biol. 2009;168:521–526. - PubMed
-
- Allen Institute for Brain Science. Allen brain atlas. Seattle, WA: Allen Institute for Brain Science; 2009. Available at http://www.brain-map.org.
-
- Bremer M, Fröb F, Kichko T, Reeh P, Tamm ER, Suter U, Wegner M. Sox10 is required for Schwann-cell homeostasis and myelin maintenance in the adult peripheral nerve. Glia. 2011;59:1022–1032. - PubMed
-
- Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2:419–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
