Brefeldin A-inhibited guanine exchange factor 2 regulates filamin A phosphorylation and neuronal migration
- PMID: 22956851
- PMCID: PMC3478955
- DOI: 10.1523/JNEUROSCI.1063-12.2012
Brefeldin A-inhibited guanine exchange factor 2 regulates filamin A phosphorylation and neuronal migration
Abstract
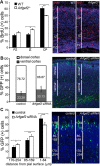
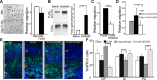
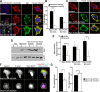
Periventricular heterotopia (PH) is a human malformation of cortical development associated with gene mutations in ADP-ribosylation factor guanine exchange factor 2 (ARFGEF2 encodes for Big2 protein) and Filamin A (FLNA). PH is thought to derive from neuroependymal disruption, but the extent to which neuronal migration contributes to this phenotype is unknown. Here, we show that Arfgef2 null mice develop PH and exhibit impaired neural migration with increased protein expression for both FlnA and phosphoFlnA at Ser2152. Big2 physically interacts with FlnA and overexpression of phosphomimetic Ser2512 FLNA impairs neuronal migration. FlnA phosphorylation directs FlnA localization toward the cell cytoplasm, diminishes its binding affinity to actin skeleton, and alters the number and size of paxillin focal adhesions. Collectively, our results demonstrate a molecular mechanism whereby Big2 inhibition promotes phosphoFlnA (Ser2152) expression, and increased phosphoFlnA impairs its actin binding affinity and the distribution of focal adhesions, thereby disrupting cell intrinsic neuronal migration.
Figures



Similar articles
-
Filamin A regulates neuronal migration through brefeldin A-inhibited guanine exchange factor 2-dependent Arf1 activation.J Neurosci. 2013 Oct 2;33(40):15735-46. doi: 10.1523/JNEUROSCI.1939-13.2013. J Neurosci. 2013. PMID: 24089482 Free PMC article.
-
Overlapping expression of ARFGEF2 and Filamin A in the neuroependymal lining of the lateral ventricles: insights into the cause of periventricular heterotopia.J Comp Neurol. 2006 Jan 20;494(3):476-84. doi: 10.1002/cne.20806. J Comp Neurol. 2006. PMID: 16320251
-
Disruption of neural progenitors along the ventricular and subventricular zones in periventricular heterotopia.Hum Mol Genet. 2009 Feb 1;18(3):497-516. doi: 10.1093/hmg/ddn377. Epub 2008 Nov 7. Hum Mol Genet. 2009. PMID: 18996916 Free PMC article.
-
Trouble making the first move: interpreting arrested neuronal migration in the cerebral cortex.Trends Neurosci. 2008 Feb;31(2):54-61. doi: 10.1016/j.tins.2007.11.009. Epub 2008 Jan 16. Trends Neurosci. 2008. PMID: 18201775 Review.
-
Filamin A: phenotypic diversity.Curr Opin Genet Dev. 2005 Jun;15(3):301-7. doi: 10.1016/j.gde.2005.04.001. Curr Opin Genet Dev. 2005. PMID: 15917206 Review.
Cited by
-
A Novel Mechanism Regulating Dopamine Receptor Type 2 Signal Transduction in Pituitary Tumoral Cells: The Role of cAMP/PKA-Induced Filamin A Phosphorylation.Front Endocrinol (Lausanne). 2021 Feb 16;11:611752. doi: 10.3389/fendo.2020.611752. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33664708 Free PMC article.
-
A mechanism of global shape-dependent recognition and phosphorylation of filamin by protein kinase A.J Biol Chem. 2015 Mar 27;290(13):8527-38. doi: 10.1074/jbc.M114.633446. Epub 2015 Feb 9. J Biol Chem. 2015. PMID: 25666618 Free PMC article.
-
Filamin A- and formin 2-dependent endocytosis regulates proliferation via the canonical Wnt pathway.Development. 2016 Dec 1;143(23):4509-4520. doi: 10.1242/dev.139295. Epub 2016 Oct 27. Development. 2016. PMID: 27789627 Free PMC article.
-
G protein-coupled receptors directly bind filamin A with high affinity and promote filamin phosphorylation.Biochemistry. 2015 Nov 10;54(44):6673-83. doi: 10.1021/acs.biochem.5b00975. Epub 2015 Nov 2. Biochemistry. 2015. PMID: 26460884 Free PMC article.
-
Small Rho-GTPases and cortical malformations: fine-tuning the cytoskeleton stability.Small GTPases. 2013 Jan-Mar;4(1):51-6. doi: 10.4161/sgtp.23093. Small GTPases. 2013. PMID: 23524873 Free PMC article.
References
-
- Achstetter T, Franzusoff A, Field C, Schekman R. SEC7 encodes an unusual, high molecular weight protein required for membrane traffic from the yeast Golgi apparatus. J Biol Chem. 1988;263:11711–11717. - PubMed
-
- Charych EI, Yu W, Miralles CP, Serwanski DR, Li X, Rubio M, De Blas AL. The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J Neurochem. 2004;90:173–189. - PubMed
-
- D'Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. - PubMed
-
- Faux CH, Parnavelas JG. The role of intracellular calcium and RhoA in neuronal migration. Sci STKE. 2007;2007:pe62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
