Nanotheranostics--a review of recent publications
- PMID: 22956869
- PMCID: PMC3431969
- DOI: 10.2147/IJN.S33065
Nanotheranostics--a review of recent publications
Abstract
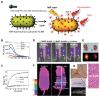
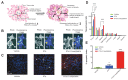
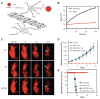
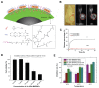
Theranostics is referred to as a treatment strategy that combines therapeutics with diagnostics, aiming to monitor the response to treatment and increase drug efficacy and safety, which would be a key part of personalized medicine and require considerable advances in predictive medicine. Theranostics associates with both a diagnosis that tests patients for possible reactions to taking new medication and targeted drug delivery based on the test results. Emerging nanotechnology provides a great deal of opportunity to design and develop such combination agents, permitting the delivery of therapeutics and concurrently allowing the detection modality to be used not only before or after but also throughout the entire treatment regimen. The introduction of nanotheranostics into routine health care has still a long way to go, since evaluations on cytotoxicity, genotoxicity, and immunotoxicity of prospective nanotheranostics, demonstration of cost-effectiveness, and availability of appropriate accessible testing systems are still required. An extensive review, from a chemistry point of view, of the recent development of nanotheranostics and its in vitro and in vivo applications are herein presented.
Keywords: diagnostics; nanomaterial; theranostics; therapeutics.
Figures

References
-
- Funkhouser J. Reinventing pharma: the theranostic revolution. Curr Drug Discov. 2002;2:17–19.
-
- Park K, Lee S, Kang E, Kim K, Choi K, Kwon IC. New generation of multifunctional nanoparticles for cancer imaging and therapy. Adv Funct Mater. 2009;19(10):1553–1566.
-
- Cai W, Chen X. Nanoplatforms for targeted molecular imaging in living subjects. Small. 2007;3(11):1840–1854. - PubMed
-
- Zhang Z, Jia J, Lai Y, Ma Y, Weng J, Sun L. Conjugating folic acid to gold nanoparticles through glutathione for targeting and detecting cancer cells. Bioorg Med Chem. 2010;18(15):5528–5534. - PubMed
-
- Heo DN, Yang DH, Moon HJ, et al. Gold nanoparticles surface- functionalized with paclitaxel drug and biotin receptor as theranostic agents for cancer therapy. Biomaterials. 2012;33(3):856–866. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

