Towards real-time detection of tumor margins using photothermal imaging of immune-targeted gold nanoparticles
- PMID: 22956871
- PMCID: PMC3431967
- DOI: 10.2147/IJN.S34157
Towards real-time detection of tumor margins using photothermal imaging of immune-targeted gold nanoparticles
Abstract
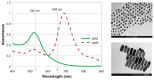
Background: One of the critical problems in cancer management is local recurrence of disease. Between 20% and 30% of patients who undergo tumor resection surgery require reoperation due to incomplete excision. Currently, there are no validated methods for intraoperative tumor margin detection. In the present work, we demonstrate the potential use of gold nanoparticles (GNPs) as a novel contrast agent for photothermal molecular imaging of cancer.
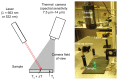
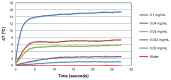
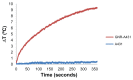
Methods: Phantoms containing different concentrations of GNPs were irradiated with continuous-wave laser and measured with a thermal imaging camera which detected the temperature field of the irradiated phantoms.
Results: The results clearly demonstrate the ability to distinguish between cancerous cells specifically targeted with GNPs and normal cells. This technique, which allows highly sensitive discrimination between adjacent low GNP concentrations, will allow tumor margin detection while the temperature increases by only a few degrees Celsius (for GNPs in relevant biological concentrations).
Conclusion: We expect this real-time intraoperative imaging technique to assist surgeons in determining clear tumor margins and to maximize the extent of tumor resection while sparing normal background tissue.
Keywords: gold nanoparticles; molecular imaging; photothermal imaging.
Figures


Similar articles
-
Improved Margins Detection of Regions Enriched with Gold Nanoparticles inside Biological Phantom.Materials (Basel). 2017 Feb 20;10(2):203. doi: 10.3390/ma10020203. Materials (Basel). 2017. PMID: 28772563 Free PMC article.
-
Targeted gold nanoparticles enable molecular CT imaging of cancer: an in vivo study.Int J Nanomedicine. 2011;6:2859-64. doi: 10.2147/IJN.S25446. Epub 2011 Nov 11. Int J Nanomedicine. 2011. PMID: 22131831 Free PMC article.
-
Dynamic In Vivo X-ray Fluorescence Imaging of Gold in Living Mice Exposed to Gold Nanoparticles.IEEE Trans Med Imaging. 2020 Feb;39(2):526-533. doi: 10.1109/TMI.2019.2932014. Epub 2019 Jul 30. IEEE Trans Med Imaging. 2020. PMID: 31380749
-
Gold nanoparticles in ophthalmology.Med Res Rev. 2019 Jan;39(1):302-327. doi: 10.1002/med.21509. Epub 2018 May 16. Med Res Rev. 2019. PMID: 29766541 Review.
-
Recent Advances of Gold Nanoparticles in Biomedical Applications: State of the Art.Cell Biochem Biophys. 2019 Jun;77(2):123-137. doi: 10.1007/s12013-018-0863-4. Epub 2018 Dec 20. Cell Biochem Biophys. 2019. PMID: 30570696 Review.
Cited by
-
Gold nanoparticles allow detection of early-stage edema in mice via computed tomography imaging.Int J Nanomedicine. 2015 Jun 2;10:3803-14. doi: 10.2147/IJN.S77383. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26082631 Free PMC article.
-
The effect of nanoparticle size on the probability to cross the blood-brain barrier: an in-vitro endothelial cell model.J Nanobiotechnology. 2015 Mar 4;13:19. doi: 10.1186/s12951-015-0075-7. J Nanobiotechnology. 2015. PMID: 25880565 Free PMC article.
-
Optimization of Gold Nanorod Features for the Enhanced Performance of Plasmonic Nanocavity Arrays.ACS Omega. 2021 Oct 22;6(43):29071-29077. doi: 10.1021/acsomega.1c04301. eCollection 2021 Nov 2. ACS Omega. 2021. PMID: 34746596 Free PMC article.
-
Targeted Magnetic Nanoparticles for Mechanical Lysis of Tumor Cells by Low-Amplitude Alternating Magnetic Field.Materials (Basel). 2016 Nov 22;9(11):943. doi: 10.3390/ma9110943. Materials (Basel). 2016. PMID: 28774062 Free PMC article.
-
Plasmonic Nanoparticle-Enhanced Optical Techniques for Cancer Biomarker Sensing.Biosensors (Basel). 2023 Nov 8;13(11):977. doi: 10.3390/bios13110977. Biosensors (Basel). 2023. PMID: 37998152 Free PMC article. Review.
References
-
- World Health Organization. [Accessed July 23, 2012];Cancer Fact Sheet. 2009 297 Available from: http://www.who.int/mediacentre/factsheets/fs297/en/index.html.
-
- Smitt MC, Nowels K, Carlson RW, Jeffrey SS. Predictors of reexcision findings and recurrence after breast conservation. Int J Radiat Oncol Biol Phys. 2003;57(4):979–985. - PubMed
-
- Menes T, Tartter P, Bleiweiss I, Godbold J, Estabrook A, Smith S. The consequence of multiple re-excisions to obtain clear lumpectomy margins in breast cancer patients. Ann Surg Oncol. 2005;12(11):881–885. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

