Long-term tolerability of once-monthly injectable paliperidone palmitate in subjects with recently diagnosed schizophrenia
- PMID: 22956873
- PMCID: PMC3431970
- DOI: 10.2147/NDT.S32581
Long-term tolerability of once-monthly injectable paliperidone palmitate in subjects with recently diagnosed schizophrenia
Abstract
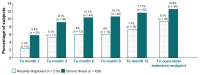
Background: A post hoc analysis from a multiphase trial with open-label transition and maintenance phases, a double-blind relapse prevention phase, and an optional open-label extension examined the long-term tolerability with continuous once-monthly injectable paliperidone palmitate 39, 78, 117, or 156 mg (25, 50, 75, or 100 mg equivalents [mg eq] of paliperidone) in subjects with recently diagnosed (≤5 years; n = 216) versus chronic illness (>5 years; n = 429) schizophrenia.
Methods: Adverse events reported at a ≥2% margin between subgroups were identified. Relative risks (in the recently diagnosed compared with the chronically ill) and 95% confidence intervals (CI) were determined, and CI not including 1 were considered potentially significant.
Results: In both subgroups, the mean monthly dose was 109 mg (69.9 mg eq). Continuous mean exposures were 333.9 ± 271.9 and 308.7 ± 278.3 days in the recently diagnosed and chronic illness subgroups, respectively. Using the criteria outlined in the methods, nasopharyngitis was a potentially significant event reported in more chronically ill than recently diagnosed subjects at months 6, 9, 12, and endpoint (7.2% versus 2.8%; relative risk 0.384; 95% CI 0.163-0.907). Influenza (2.8% versus 0.7%; relative risk 3.9; 95% CI 1.003-15.730) and amenorrhea (3.2% versus 0.9%; relative risk 3.476; 95% CI 1.029-11.744) at endpoint were potentially significant events in more recently diagnosed than chronically ill subjects. Mean weight changes, sedation/somnolence, any extrapyramidal symptom-related or glucose-related events were generally similar between the groups. The mean prolactin level increased in both sexes in both subgroups (changes from baseline of +41.8 ng/mL and +26.5 ng/mL in recently diagnosed and chronic illness females and +12.3 ng/mL and +15.1 ng/mL in recently diagnosed and chronic illness males, respectively), and were higher in females with recently diagnosed illness than in females who were chronically ill (P = 0.0002 at endpoint). Prolactin-related events were reported by 7.9% of recently diagnosed subjects with schizophrenia and 3.5% of those who were chronically ill.
Conclusion: The long-term tolerability of paliperidone palmitate was generally similar in recently diagnosed schizophrenia subjects and those with more chronic illness, with the exception of some prolactin-related measures.
Keywords: early illness; long-acting antipsychotic; paliperidone palmitate; recently diagnosed; schizophrenia.
Figures


References
-
- Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2006;4:CD004718. - PubMed
-
- Kevashan MS, Amirsadri A. Early intervention in schizophrenia: current and future perspectives. Curr Psychiatry Rep. 2007;9:325–328. - PubMed
-
- Lieberman JA. Is schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspective. Biol Psychiatry. 1999;46:729–739. - PubMed
-
- Lieberman JA, Perkins D, Belger A, et al. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50:884–897. - PubMed
LinkOut - more resources
Full Text Sources

