Efficacy of interspinous device versus surgical decompression in the treatment of lumbar spinal stenosis: a modified network analysis
- PMID: 22956936
- PMCID: PMC3427972
- DOI: 10.1055/s-0030-1267086
Efficacy of interspinous device versus surgical decompression in the treatment of lumbar spinal stenosis: a modified network analysis
Abstract
Study design: Systematic review using a modified network analysis.
Objectives: To compare the effectiveness and morbidity of interspinous-device placement versus surgical decompression for the treatment of lumbar spinal stenosis.
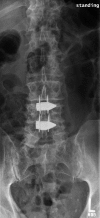
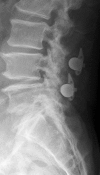
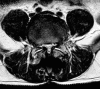
Summary: Traditionally, the most effective treatment for degenerative lumbar spinal stenosis is through surgical decompression. Recently, interspinous devices have been used in lieu of standard laminectomy.
Methods: A review of the English-language literature was undertaken for articles published between 1970 and March 2010. Electronic databases and reference lists of key articles were searched to identify studies comparing surgical decompression with interspinous-device placement for the treatment of lumbar spinal stenosis. First, studies making the direct comparison (cohort or randomized trials) were searched. Second, randomized controlled trials (RCTs) comparing each treatment to conservative management were searched to allow for an indirect comparison through a modified network analysis approach. Comparison studies involving simultaneous decompression with placement of an interspinous device were not included. Studies that did not have a comparison group were not included since a treatment effect could not be calculated. Two independent reviewers assessed the strength of evidence using the GRADE criteria assessing quality, quantity, and consistency of results. The strengths of evidence for indirect comparisons were downgraded. Disagreements were resolved by consensus.
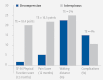
Results: We identified five studies meeting our inclusion criteria. No RCTs or cohort studies were identified that made the direct comparison of interspinous-device placement with surgical decompression. For the indirect comparison, three RCTs compared surgical decompression to conservative management and two RCTs compared interspinous-device placement to conservative management. There was low evidence supporting greater treatment effects for interspinous-device placement compared to decompression for disability and pain outcomes at 12 months. There was low evidence demonstrating little to no difference in treatment effects between the groups for walking distance and complication rates.
Conclusion: The indirect treatment effect for disability and pain favors the interspinous device compared to decompression. The low evidence suggests that any further research is very likely to have an important impact on the confidence in the estimate of effect and is likely to change the estimate. No significant treatment effect differences were observed for postoperative walking distance improvement or complication rates; however, findings should be considered with caution because of indirect comparisons and short follow-up periods.
Conflict of interest statement
This systematic review was funded by AOSpine
Figures



References
-
- Malmivaara A, Slätis P, Heliövaara M. et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine. 2007;32(1):1–8. - PubMed

