Next generation orthopaedic implants by additive manufacturing using electron beam melting
- PMID: 22956957
- PMCID: PMC3432366
- DOI: 10.1155/2012/245727
Next generation orthopaedic implants by additive manufacturing using electron beam melting
Abstract
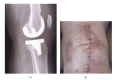
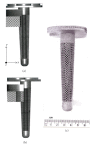
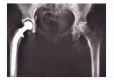
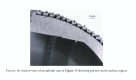
This paper presents some examples of knee and hip implant components containing porous structures and fabricated in monolithic forms utilizing electron beam melting (EBM). In addition, utilizing stiffness or relative stiffness versus relative density design plots for open-cellular structures (mesh and foam components) of Ti-6Al-4V and Co-29Cr-6Mo alloy fabricated by EBM, it is demonstrated that stiffness-compatible implants can be fabricated for optimal stress shielding for bone regimes as well as bone cell ingrowth. Implications for the fabrication of patient-specific, monolithic, multifunctional orthopaedic implants using EBM are described along with microstructures and mechanical properties characteristic of both Ti-6Al-4V and Co-29Cr-6Mo alloy prototypes, including both solid and open-cellular prototypes manufactured by additive manufacturing (AM) using EBM.
Figures












References
-
- Bronzino JD, editor. The Biomedical Engineering Handbook. Vol. 1. Boca Raton, Fla, USA: CRC Press; 2000.
-
- Christel P, Meunier A, Lee AJC, editors. Biological and Biomechanical Performance of Biomaterials. New York, NY, USA: Elsevier; 1986.
-
- Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Engineering. 2001;7(6):679–689. - PubMed
-
- Cahoon JR. On the corrosion products of orthopedic implants. Journal of Biomedical Materials Research. 1973;7(4):375–383. - PubMed
-
- Ryan G, Pandit A, Apatsidis DP. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials. 2006;27(13):2651–2670. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

