Universal access to HIV treatment versus universal 'test and treat': transmission, drug resistance & treatment costs
- PMID: 22957012
- PMCID: PMC3434222
- DOI: 10.1371/journal.pone.0041212
Universal access to HIV treatment versus universal 'test and treat': transmission, drug resistance & treatment costs
Abstract
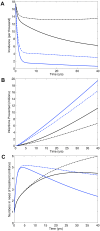
In South Africa (SA) universal access to treatment for HIV-infected individuals in need has yet to be achieved. Currently ~1 million receive treatment, but an additional 1.6 million are in need. It is being debated whether to use a universal 'test and treat' (T&T) strategy to try to eliminate HIV in SA; treatment reduces infectivity and hence transmission. Under a T&T strategy all HIV-infected individuals would receive treatment whether in need or not. This would require treating 5 million individuals almost immediately and providing treatment for several decades. We use a validated mathematical model to predict impact and costs of: (i) a universal T&T strategy and (ii) achieving universal access to treatment. Using modeling the WHO has predicted a universal T&T strategy in SA would eliminate HIV within a decade, and (after 40 years) cost ~$10 billion less than achieving universal access. In contrast, we predict a universal T&T strategy in SA could eliminate HIV, but take 40 years and cost ~$12 billion more than achieving universal access. We determine the difference in predictions is because the WHO has under-estimated survival time on treatment and ignored the risk of resistance. We predict, after 20 years, ~2 million individuals would need second-line regimens if a universal T&T strategy is implemented versus ~1.5 million if universal access is achieved. Costs need to be realistically estimated and multiple evaluation criteria used to compare 'treatment as prevention' with other prevention strategies. Before implementing a universal T&T strategy, which may not be sustainable, we recommend striving to achieve universal access to treatment as quickly as possible. We predict achieving universal access to treatment would be a very effective 'treatment as prevention' approach and bring the HIV epidemic in SA close to elimination, preventing ~4 million infections after 20 years and ~11 million after 40 years.
Conflict of interest statement
Figures




References
-
- Cohen MS, Mastro TD, Cates W (2009) Universal voluntary HIV testing and immediate antiretroviral therapy. Lancet 373: 1077 author reply 1080–1071. - PubMed
-
- Dieffenbach CW, Fauci AS (2009) Universal voluntary testing and treatment for prevention of HIV transmission. JAMA 301: 2380–2382. - PubMed
-
- Garnett GP, Baggaley RF (2009) Treating our way out of the HIV pandemic: could we, would we, should we? Lancet 373: 9–11. - PubMed
-
- Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG (2009) Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 373: 48–57. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

