Specificity determinants of the silkworm moth sex pheromone
- PMID: 22957053
- PMCID: PMC3434217
- DOI: 10.1371/journal.pone.0044190
Specificity determinants of the silkworm moth sex pheromone
Abstract
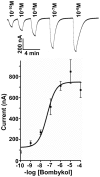
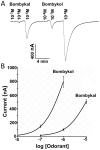
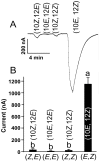
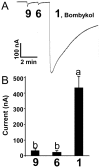
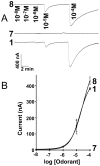
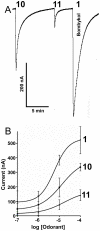
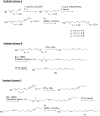
The insect olfactory system, particularly the peripheral sensory system for sex pheromone reception in male moths, is highly selective, but specificity determinants at the receptor level are hitherto unknown. Using the Xenopus oocyte recording system, we conducted a thorough structure-activity relationship study with the sex pheromone receptor of the silkworm moth, Bombyx mori, BmorOR1. When co-expressed with the obligatory odorant receptor co-receptor (BmorOrco), BmorOR1 responded in a dose-dependent fashion to both bombykol and its related aldehyde, bombykal, but the threshold of the latter was about one order of magnitude higher. Solubilizing these ligands with a pheromone-binding protein (BmorPBP1) did not enhance selectivity. By contrast, both ligands were trapped by BmorPBP1 leading to dramatically reduced responses. The silkworm moth pheromone receptor was highly selective towards the stereochemistry of the conjugated diene, with robust response to the natural (10E,12Z)-isomer and very little or no response to the other three isomers. Shifting the conjugated diene towards the functional group or elongating the carbon chain rendered these molecules completely inactive. In contrast, an analogue shortened by two omega carbons elicited the same or slightly higher responses than bombykol. Flexibility of the saturated C1-C9 moiety is important for function as addition of a double or triple bond in position 4 led to reduced responses. The ligand is hypothesized to be accommodated by a large hydrophobic cavity within the helical bundle of transmembrane domains.
Conflict of interest statement
Figures




References
-
- Butenandt A, Beckmann R, Stamm D, Hecker E (1959) Uber den sexual lockstoff des seidenspinners Bombyx mori - Reindarstellung und konstitution. Zeitschrift Fur Naturforschung Part B-Chemie Biochemie Biophysik Biologie Und Verwandten Gebiete 14: 283–284.
-
- Kaissling KE (1987) Wright lectures on insect olfaction. British Columbia, Canada: Simon Fraser University.
-
- Kaissling K-E, Priesner E (1970) Die Riechschwelle des Seidenspinners. Naturwissenschaften 57: 23–28. - PubMed
-
- Nakagawa T, Sakurai T, Nishioka T, Touhara K (2005) Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science 307: 1638–1642. - PubMed
-
- Grosse-Wilde E, Svatos A, Krieger J (2006) A pheromone-binding protein mediates the bombykol-induced activation of a pheromone receptor in vitro. Chem Senses 31: 547–555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

