Expression of aromatase in radial glial cells in the brain of the Japanese eel provides insight into the evolution of the cyp191a gene in Actinopterygians
- PMID: 22957105
- PMCID: PMC3434150
- DOI: 10.1371/journal.pone.0044750
Expression of aromatase in radial glial cells in the brain of the Japanese eel provides insight into the evolution of the cyp191a gene in Actinopterygians
Abstract
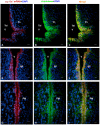
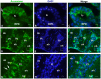
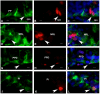
The cyp19a1 gene that encodes aromatase, the only enzyme permitting conversion of C19 aromatizable androgens into estrogens, is present as a single copy in the genome of most vertebrate species, except in teleosts in which it has been duplicated. This study aimed at investigating the brain expression of a cyp19a1 gene expressed in both gonad and brain of Japanese eel, a basal teleost. By means of immunohistochemistry and in situ hybridization, we show that cyp19a1 is expressed only in radial glial cells of the brain and in pituitary cells. Treatments with salmon pituitary homogenates (female) or human chorionic gonadotrophin (male), known to turn on steroid production in immature eels, strongly stimulated cyp19a1 messenger and protein expression in radial glial cells and pituitary cells. Using double staining studies, we also showed that aromatase-expressing radial glial cells exhibit proliferative activity in both the brain and the pituitary. Altogether, these data indicate that brain and pituitary expression of Japanese eel cyp19a1 exhibits characteristics similar to those reported for the brain specific cyp19a1b gene in teleosts having duplicated cyp19a1 genes. This supports the hypothesis that, despite the fact that eels also underwent the teleost specific genome duplication, they have a single cyp19a1 expressed in both brain and gonad. Such data also suggest that the intriguing features of brain aromatase expression in teleost fishes were not gained after the whole genome duplication and may reflect properties of the cyp19a1 gene of ancestral Actinopterygians.
Conflict of interest statement
Figures





Similar articles
-
Basal teleosts provide new insights into the evolutionary history of teleost-duplicated aromatase.Gen Comp Endocrinol. 2020 May 15;291:113395. doi: 10.1016/j.ygcen.2020.113395. Epub 2020 Jan 23. Gen Comp Endocrinol. 2020. PMID: 31981691
-
Differential regulation of the expression of cytochrome P450 aromatase, estrogen and androgen receptor subtypes in the brain-pituitary-ovarian axis of the Japanese eel (Anguilla japonica) reveals steroid dependent and independent mechanisms.Gen Comp Endocrinol. 2012 Jan 1;175(1):163-72. doi: 10.1016/j.ygcen.2011.11.005. Epub 2011 Nov 15. Gen Comp Endocrinol. 2012. PMID: 22107840
-
Genes encoding aromatases in teleosts: evolution and expression regulation.Gen Comp Endocrinol. 2014 Sep 1;205:151-8. doi: 10.1016/j.ygcen.2014.05.008. Epub 2014 May 20. Gen Comp Endocrinol. 2014. PMID: 24859258 Review.
-
Differential expression of neural and gonadal aromatase enzymatic activities in relation to gonadal development in Japanese eel, Anguilla japonica.J Exp Zool A Comp Exp Biol. 2005 Sep 1;303(9):802-12. doi: 10.1002/jez.a.194. J Exp Zool A Comp Exp Biol. 2005. PMID: 16106412
-
Aromatase in the brain of teleost fish: expression, regulation and putative functions.Front Neuroendocrinol. 2010 Apr;31(2):172-92. doi: 10.1016/j.yfrne.2010.01.003. Epub 2010 Jan 29. Front Neuroendocrinol. 2010. PMID: 20116395 Review.
Cited by
-
Post-proliferative immature radial glial cells female-specifically express aromatase in the medaka optic tectum.PLoS One. 2013 Sep 3;8(9):e73663. doi: 10.1371/journal.pone.0073663. eCollection 2013. PLoS One. 2013. PMID: 24019933 Free PMC article.
-
Expression and sequence evolution of aromatase cyp19a1 and other sexual development genes in East African cichlid fishes.Mol Biol Evol. 2013 Oct;30(10):2268-85. doi: 10.1093/molbev/mst124. Epub 2013 Jul 24. Mol Biol Evol. 2013. PMID: 23883521 Free PMC article.
-
Post-Traumatic Expressions of Aromatase B, Glutamine Synthetase, and Cystathionine-Beta-Synthase in the Cerebellum of Juvenile Chum Salmon, Oncorhynchus keta.Int J Mol Sci. 2024 Mar 14;25(6):3299. doi: 10.3390/ijms25063299. Int J Mol Sci. 2024. PMID: 38542274 Free PMC article.
-
Direct and Indirect Effects of Sex Steroids on Gonadotrope Cell Plasticity in the Teleost Fish Pituitary.Front Endocrinol (Lausanne). 2020 Dec 7;11:605068. doi: 10.3389/fendo.2020.605068. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33365013 Free PMC article. Review.
-
Transcriptomic analysis of melatonin on the mechanism of embryonic gonadal development in female Jilin white geese.Poult Sci. 2025 Jan;104(1):104527. doi: 10.1016/j.psj.2024.104527. Epub 2024 Nov 10. Poult Sci. 2025. PMID: 39566171 Free PMC article.
References
-
- Ohno S (1970) Evolution by Gene Duplication. London: Allen and Unwin,.
-
- Ravi V, Venkatesh B (2008) Rapidly evolving fish genomes and teleost diversity. Curr Opin Genet Dev 18: 544–550. - PubMed
-
- Amores A, Force A, Yan YL, Joly L, Amemiya C, et al. (1998) Zebrafish hox clusters and vertebrate genome evolution. Science 282: 1711–1714. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

