A novel Myosin essential light chain mutation causes hypertrophic cardiomyopathy with late onset and low expressivity
- PMID: 22957257
- PMCID: PMC3432877
- DOI: 10.1155/2012/685108
A novel Myosin essential light chain mutation causes hypertrophic cardiomyopathy with late onset and low expressivity
Abstract
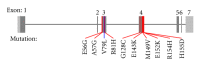
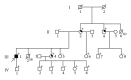
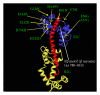
Hypertrophic cardiomyopathy (HCM) is caused by mutations in genes encoding sarcomere proteins. Mutations in MYL3, encoding the essential light chain of myosin, are rare and have been associated with sudden death. Both recessive and dominant patterns of inheritance have been suggested. We studied a large family with a 38-year-old asymptomatic HCM-affected male referred because of a murmur. The patient had HCM with left ventricular hypertrophy (max WT 21 mm), a resting left ventricular outflow gradient of 36 mm Hg, and left atrial dilation (54 mm). Genotyping revealed heterozygosity for a novel missense mutation, p.V79I, in MYL3. The mutation was not found in 300 controls, and the patient had no mutations in 10 sarcomere genes. Cascade screening revealed a further nine heterozygote mutation carriers, three of whom had ECG and/or echocardiographic abnormalities but did not fulfil diagnostic criteria for HCM. The penetrance, if we consider this borderline HCM the phenotype of the p.V79I mutation, was 40%, but the mean age of the nonpenetrant mutation carriers is 15, while the mean age of the penetrant mutation carriers is 47. The mutation affects a conserved valine replacing it with a larger isoleucine residue in the region of contact between the light chain and the myosin lever arm. In conclusion, MYL3 mutations can present with low expressivity and late onset.
Figures


References
-
- Erdmann J, Daehmlow S, Wischke S, et al. Mutation spectrum in a large cohort of unrelated consecutive patients with hypertrophic cardiomyopathy. Clinical Genetics. 2003;64(4):339–349. - PubMed
-
- Niimura H, Patton KK, McKenna WJ, et al. Sarcomere protein gene mutations in hypertrophic cardiomyopathy of the elderly. Circulation. 2002;105(4):446–451. - PubMed
-
- Richard P, Charron P, Carrier L, et al. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107(17):2227–2232. - PubMed
-
- Andersen PS, Havndrup O, Hougs L, et al. Diagnostic yield, interpretation, and clinical utility of mutation screening of sarcomere encoding genes in Danish hypertrophic cardiomyopathy patients and relatives. Human Mutation. 2009;30(3):363–370. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

