BRCA1 proteins regulate growth of ovarian cancer cells by tethering Ubc9
- PMID: 22957306
- PMCID: PMC3433105
BRCA1 proteins regulate growth of ovarian cancer cells by tethering Ubc9
Abstract
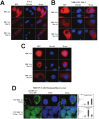
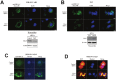
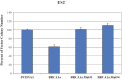
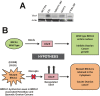
Mutation in the BRCA1 gene is associated with increased risk for hereditary breast and ovarian cancers. In sporadic ovarian tumors, BRCA1 dysfunction is thought to be common. BRCA1 is a nuclear-cytoplasm shuttling protein. Our group has previously reported that BRCA1 proteins, unlike K109R and cancer-predisposing mutant C61G BRCA1 proteins, bind the sole SUMO E2-conjugating enzyme Ubc9. In this study, we examined the result of altered Ubc9 binding and knockdown on the sub-cellular localization and growth inhibitory function of BRCA1 proteins in ovarian cancer cells. Using live imaging of YFP, RFP-tagged BRCA1 and BRCA1a proteins, our results show enhanced cytoplasmic localization of K109R and C61G mutant BRCA1 proteins in ES-2, NIHOVCAR3 and UWB 1.289 ovarian cancer cells. Down-regulation of Ubc9 in ovarian cancer cells using Ubc9 siRNA resulted in cytoplasmic localization of BRCA1 and BRCA1a proteins. These mutant BRCA1a proteins were impaired in their capacity to inhibit growth of ES-2 ovarian cancer cells. Several ovarian cancer cells, including a BRCA1-null ovarian cancer cell line, showed higher levels of expression of Ubc9. This is the first study demonstrating the physiological link between loss of Ubc9 binding and loss of growth suppression of disease-associated mutant BRCA1a proteins in ovarian cancer cells. BRCA1, by turning off or on Ubc9 binding, regulates growth of ovarian cancers.
Keywords: BRCA1; BRCA1a; Growth suppression; Ovarian cancer; RING domain mutants; Ubc9; nuclear import.
Figures
Similar articles
-
BRCA1 Mutation Leads to Deregulated Ubc9 Levels which Triggers Proliferation and Migration of Patient-Derived High Grade Serous Ovarian Cancer and Triple Negative Breast Cancer Cells.Int J Chronic Dis Ther. 2016;2(3):31-38. Epub 2016 Jun 23. Int J Chronic Dis Ther. 2016. PMID: 28164176 Free PMC article.
-
A Novel Pathway that Links Caveolin-1 Down-Regulation to BRCA1 Dysfunction in Serous Epithelial Ovarian Cancer Cells.Enliven Chall Cancer Detect Ther. 2014;1(1):004. doi: 10.18650/2376-046x.11004. Enliven Chall Cancer Detect Ther. 2014. PMID: 25594072 Free PMC article.
-
Ubc9 mediates nuclear localization and growth suppression of BRCA1 and BRCA1a proteins.J Cell Physiol. 2011 Dec;226(12):3355-67. doi: 10.1002/jcp.22695. J Cell Physiol. 2011. PMID: 21344391 Free PMC article.
-
Breast cancer genes: therapeutic strategies.Ann N Y Acad Sci. 1997 Dec 29;833:34-41. doi: 10.1111/j.1749-6632.1997.tb48590.x. Ann N Y Acad Sci. 1997. PMID: 9616738 Review.
-
Targeting Ubc9 for cancer therapy.Expert Opin Ther Targets. 2005 Dec;9(6):1203-16. doi: 10.1517/14728222.9.6.1203. Expert Opin Ther Targets. 2005. PMID: 16300471 Review.
Cited by
-
BRCA1 Mutation Leads to Deregulated Ubc9 Levels which Triggers Proliferation and Migration of Patient-Derived High Grade Serous Ovarian Cancer and Triple Negative Breast Cancer Cells.Int J Chronic Dis Ther. 2016;2(3):31-38. Epub 2016 Jun 23. Int J Chronic Dis Ther. 2016. PMID: 28164176 Free PMC article.
-
Crossing epigenetic frontiers: the intersection of novel histone modifications and diseases.Signal Transduct Target Ther. 2024 Sep 16;9(1):232. doi: 10.1038/s41392-024-01918-w. Signal Transduct Target Ther. 2024. PMID: 39278916 Free PMC article. Review.
-
A Novel Pathway that Links Caveolin-1 Down-Regulation to BRCA1 Dysfunction in Serous Epithelial Ovarian Cancer Cells.Enliven Chall Cancer Detect Ther. 2014;1(1):004. doi: 10.18650/2376-046x.11004. Enliven Chall Cancer Detect Ther. 2014. PMID: 25594072 Free PMC article.
-
Epithelial ovarian cancer: An overview.World J Transl Med. 2014 Apr 12;3(1):1-8. doi: 10.5528/wjtm.v3.i1.1. World J Transl Med. 2014. PMID: 25525571 Free PMC article.
-
E2F1 and TFDP1 Regulate PITX1 Expression in Normal and Osteoarthritic Articular Chondrocytes.PLoS One. 2016 Nov 1;11(11):e0165951. doi: 10.1371/journal.pone.0165951. eCollection 2016. PLoS One. 2016. PMID: 27802335 Free PMC article.
References
-
- Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, Bell R, Rosenthal J, Hussey C, Tran T, Mcclure M, Frye C, Hattier T, PHELPS R, Haugen-Strano A, Katcher H, Yakumo K, Gholami Z, Shaffer D, Stone S, Bayer S, Wray C, Bogden R, Dayananth P, Ward J, Tonin P, Narod S, Bristow PK, Norris FH, Helvering L, Morrison P, Rosteck P, LAI M, Barrett JC, Lewis C, Neuhausen S, Cannon-Albright L, Goldgar D, Wiseman R, Kamb A, Skolnick MH. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. - PubMed
-
- Berchuck A, Heron KA, Carney ME, Lancaster JM, Fraser EG, Vinson VL, Deffenbaugh AM, Miron A, Marks JR, Futreal PA, Frank TS. Frequency of germline and somatic BRCA1 mutations in ovarian cancer. Clin Cancer Res. 1998;4:2433–2437. - PubMed
-
- Merajver SD, Pham TM, Caduff RF, Chen M, Poy EL, Cooney KA, Weber BL, Collins FS, Johnston C, Frank TS. Somatic mutations in the BRCA1 gene in sporadic ovarian tumours. Nat Genet. 1995;9:439–443. - PubMed
-
- Nguewa PA, Fuertes MA, Cepeda V, Alonso C, Quevedo C, Soto M, Perez JM. Poly(ADP-ribose) polymerase-1 inhibitor 3-aminobenzamide enhances apoptosis induction by platinum complexes in cisplatin-resistant tumor cells. Med Chem. 2006;2:47–53. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
