Acute and chronic shear stress differently regulate endothelial internalization of nanocarriers targeted to platelet-endothelial cell adhesion molecule-1
- PMID: 22957767
- PMCID: PMC3874124
- DOI: 10.1021/nn302687n
Acute and chronic shear stress differently regulate endothelial internalization of nanocarriers targeted to platelet-endothelial cell adhesion molecule-1
Abstract
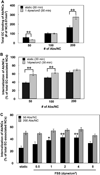
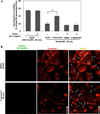
Intracellular delivery of nanocarriers (NC) is controlled by their design and target cell phenotype, microenvironment, and functional status. Endothelial cells (EC) lining the vascular lumen represent an important target for drug delivery. Endothelium in vivo is constantly or intermittently (as, for example, during ischemia-reperfusion) exposed to blood flow, which influences NC-EC interactions by changing NC transport properties, and by direct mechanical effects upon EC mechanisms involved in NC binding and uptake. EC do not internalize antibodies to marker glycoprotein PECAM(CD31), yet internalize multivalent NC coated with PECAM antibodies (anti-PECAM/NC) via a noncanonical endocytic pathway distantly related to macropinocytosis. Here we studied the effects of flow on EC uptake of anti-PECAM/NC spheres (~180 nm diameter). EC adaptation to chronic flow, manifested by cellular alignment with flow direction and formation of actin stress fibers, inhibited anti-PECAM/NC endocytosis consistent with lower rates of anti-PECAM/NC endocytosis in vivo in arterial compared to capillary vessels. Acute induction of actin stress fibers by thrombin also inhibited anti-PECAM/NC endocytosis, demonstrating that formation of actin stress fibers impedes EC endocytic machinery. In contrast, acute flow without stress fiber formation, stimulated anti-PECAM/NC endocytosis. Anti-PECAM/NC endocytosis did not correlate with the number of cell-bound particles under flow or static conditions. PECAM cytosolic tail deletion and disruption of cholesterol-rich plasmalemma domains abrogated anti-PECAM/NC endocytosis stimulation by acute flow, suggesting complex regulation of a flow-sensitive endocytic pathway in EC. The studies demonstrate the importance of the local flow microenvironment for NC uptake by the endothelium and suggest that cell culture models of nanoparticle uptake should reflect the microenvironment and phenotype of the target cells.
Conflict of interest statement
Figures





References
-
- Reilly MJ, Larsen JD, Sullivan MO. Polyplexes Traffic through Caveolae to the Golgi and Endoplasmic Reticulum en Route to the Nucleus. Mol. Pharm. 2012;9:1280–1290. - PubMed
-
- Evans CW, Fitzgerald M, Clemons TD, House MJ, Padman BS, Shaw JA, Saunders M, Harvey AR, Zdyrko B, Luzinov I, et al. Multimodal Analysis of PEI-Mediated Endocytosis of Nanoparticles in Neural Cells. ACS Nano. 2011;5:8640–8648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

