Surface sensing and lateral subcellular localization of WspA, the receptor in a chemosensory-like system leading to c-di-GMP production
- PMID: 22957788
- PMCID: PMC3501340
- DOI: 10.1111/mmi.12013
Surface sensing and lateral subcellular localization of WspA, the receptor in a chemosensory-like system leading to c-di-GMP production
Abstract
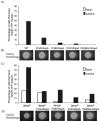
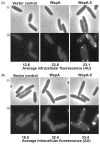
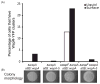
Pseudomonas aeruginosa responds to growth on agar surfaces to produce cyclic-di-GMP, which stimulates biofilm formation. This is mediated by an alternative cellular function chemotaxis-like system called Wsp. The receptor protein WspA, is bioinformatically indistinguishable from methyl-accepting chemotaxis proteins. However, unlike standard chemoreceptors, WspA does not form stable clusters at cell poles. Rather, it forms dynamic clusters at both polar and lateral subcellular locations. To begin to study the mechanism of Wsp signal transduction in response to surfaces, we carried out a structure-function study of WspA and found that its C-terminus is important for its lateral subcellular localization and function. When this region was replaced with that of a chemoreceptor for amino acids, WspA became polarly localized. In addition, introduction of mutations in the C-terminal region of WspA that rendered this protein able to form more stable receptor-receptor interactions, also resulted in a WspA protein that was less capable of activating signal transduction. Receptor chimeras with a WspA C-terminus and N-terminal periplasmic domains from chemoreceptors that sense amino acids or malate responded to surfaces to produce c-di-GMP. Thus, the amino acid sequence of the WspA periplasmic region did not need to be conserved for the Wsp system to respond to surfaces.
© 2012 Blackwell Publishing Ltd.
Figures






References
-
- Alley MR. The highly conserved domain of the Caulobacter McpA chemoreceptor is required for its polar localization. Mol Microbiol. 2001;40:1335–1343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

