Direct in situ determination of the mechanisms controlling nanoparticle nucleation and growth
- PMID: 22957797
- PMCID: PMC3482139
- DOI: 10.1021/nn303371y
Direct in situ determination of the mechanisms controlling nanoparticle nucleation and growth
Abstract
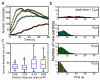
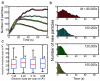
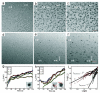
Although nanocrystal morphology is controllable using conventional colloidal synthesis, multiple characterization techniques are typically needed to determine key properties like the nucleation rate, induction time, growth rate, and the resulting morphology. Recently, researchers have demonstrated growth of nanocrystals by in situ electron beam reduction, offering direct observations of single nanocrystals and eliminating the need for multiple characterization techniques; however, they found nanocrystal morphologies consistent with two different growth mechanisms for the same electron beam parameters. Here we show that the electron beam current plays a role analogous to the concentration of reducing agent in conventional synthesis, by controlling the growth mechanism and final morphology of silver nanocrystals grown via in situ electron beam reduction. We demonstrate that low beam currents encourage reaction limited growth that yield nanocrystals with faceted structures, while higher beam currents encourage diffusion limited growth that yield spherical nanocrystals. By isolating these two growth regimes, we demonstrate a new level of control over nanocrystal morphology, regulated by the fundamental growth mechanism. We find that the induction threshold dose for nucleation is independent of the beam current, pixel dwell time, and magnification being used. Our results indicate that in situ electron microscopy data can be interpreted by classical models and that systematic dose experiments should be performed for all future in situ liquid studies to confirm the exact mechanisms underlying observations of nucleation and growth.
Figures



References
-
- Schmid G. Synthesis of Metal Nanoparticles. In: Schmid G, editor. Nanoparticles: From Theory to Application. 2nd ed. Wiley-VCH; Weinheim: 2010. pp. 228–231.
-
- Milliron DJ, Hughes SM, Cui Y, Manna L, Li JB, Wang LW, Alivisatos AP. Colloidal Nanocrystal Heterostructures with Linear and Branched Topology. Nature. 2004;430:190–195. - PubMed
-
- Peng XG, Manna L, Yang WD, Wickham J, Scher E, Kadavanich A, Alivisatos AP. Shape Control of CdSe Nanocrystals. Nature. 2000;404:59–61. - PubMed
-
- Puntes VF, Krishnan KM, Alivisatos AP. Colloidal Nanocrystal Shape and Size Control: The Case of Cobalt. Science. 2001;291:2115–2117. - PubMed
-
- Yin Y, Alivisatos AP. Colloidal Nanocrystal Synthesis and the Organic-Inorganic Interface. Nature. 2005;437:664–670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

