Expression of nuclear progesterone receptor and progesterone receptor membrane components 1 and 2 in the oviduct of cyclic and pregnant cows during the post-ovulation period
- PMID: 22958265
- PMCID: PMC3447726
- DOI: 10.1186/1477-7827-10-76
Expression of nuclear progesterone receptor and progesterone receptor membrane components 1 and 2 in the oviduct of cyclic and pregnant cows during the post-ovulation period
Abstract
Background: Progesterone (P4) may modulate oviductal functions to promote early embryo development in cattle. In addition to its nuclear receptor (PR), P4 may mediate its actions through P4 receptor membrane component 1 (PGRMC1) and its relative, PGRMC2. Two successive experiments were undertaken to characterise the expression of PR, PGRMC1 and PGRMC2 in the bovine oviduct during the post-ovulation period, and to relate their expression to the presence of an embryo, the proximity of the CL and to the region of the oviduct.
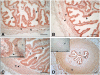
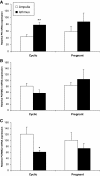
Methods: In the first experiment (Exp. I), whole oviduct sections were collected from Holstein cows at Day 1.5, Day 4 and Day 5 post-ovulation (n = 2 cows per stage). The expression of PR, PGRMC1 and PGRMC2 was studied in the ampulla and isthmus by RT-PCR, western-blot and immunohistochemistry. In Exp. II, oviduct epithelial cells were collected from cyclic and pregnant Charolais cows (n = 4 cows per status) at Day 3.5 post-ovulation and mRNA expression of PR, PGRMC1 and PGRMC2 was examined in the ampulla and isthmus by real-time quantitative PCR.
Results: In Exp. I, PR, PGRMC1 and PGRMC2 were expressed in all oviduct samples. PGRMC1 was mainly localised in the luminal epithelium whereas PR and PGRMC2 were localised in the epithelium as well as in the muscle and stroma layers of the oviduct. The expression was primarily nuclear for PR, primarily cytoplasmic for PGRMC1 and both nuclear and cytoplasmic for PGRMC2. In Exp. II, mRNA levels for PR, PGRMC1 and PGRMC2 were not affected by either the pregnancy status or the side relative to the CL. However, the expression of PR and PGRMC2 varied significantly with the region of the oviduct: PR was more highly expressed in the isthmus whereas PGRMC2 was more highly expressed in the ampulla.
Conclusions: This is the first evidence of PGRMC2 expression in the bovine oviduct. Our findings suggest that P4 regulates the functions of the bovine oviduct in a region-specific manner and through both classical and non classical pathways during the post-ovulation period.
Figures

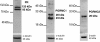


Similar articles
-
Expression of nuclear and membrane progesterone receptors in the canine oviduct during the periovulatory period.Reprod Fertil Dev. 2013;25(7):1065-76. doi: 10.1071/RD12108. Reprod Fertil Dev. 2013. PMID: 23140560
-
Local sex steroid hormone milieu in the bovine oviduct ipsilateral and contralateral to preovulatory follicle or corpus luteum during the periovulatory phase.Domest Anim Endocrinol. 2021 Jan;74:106515. doi: 10.1016/j.domaniend.2020.106515. Epub 2020 Jun 26. Domest Anim Endocrinol. 2021. PMID: 32711284
-
Expression and immunolocalization of membrane progesterone receptors in the bovine oviduct.Domest Anim Endocrinol. 2016 Apr;55:83-96. doi: 10.1016/j.domaniend.2015.12.001. Epub 2015 Dec 17. Domest Anim Endocrinol. 2016. PMID: 26774557
-
Regulation of embryo survival in cattle.Reprod Suppl. 2003;61:253-66. Reprod Suppl. 2003. PMID: 14635940 Review.
-
The critical roles of progesterone receptor (PGR) in ovulation, oocyte developmental competence and oviductal transport in mammalian reproduction.Reprod Domest Anim. 2012 Aug;47 Suppl 4:288-96. doi: 10.1111/j.1439-0531.2012.02088.x. Reprod Domest Anim. 2012. PMID: 22827383 Review.
Cited by
-
Spatiotemporal profiling of the bovine oviduct fluid proteome around the time of ovulation.Sci Rep. 2022 Mar 9;12(1):4135. doi: 10.1038/s41598-022-07929-3. Sci Rep. 2022. PMID: 35264682 Free PMC article.
-
What Do We Know about Classical and Non-Classical Progesterone Receptors in the Human Female Reproductive Tract? A Review.Int J Mol Sci. 2021 Oct 19;22(20):11278. doi: 10.3390/ijms222011278. Int J Mol Sci. 2021. PMID: 34681937 Free PMC article. Review.
-
Immunohistochemical localization of estrogen receptor alpha (ERα) in the oviduct of Indian buffalo during follicular and luteal phases of estrous cycle.Trop Anim Health Prod. 2019 Jul;51(6):1601-1609. doi: 10.1007/s11250-019-01852-y. Epub 2019 Mar 2. Trop Anim Health Prod. 2019. PMID: 30827005
-
Sex Steroid-Mediated Control of Oviductal Function in Cattle.Biology (Basel). 2018 Feb 2;7(1):15. doi: 10.3390/biology7010015. Biology (Basel). 2018. PMID: 29393864 Free PMC article. Review.
-
Effect of Steroid Hormones, Prostaglandins (E2 and F2α), Oxytocin, and Tumor Necrosis Factor Alpha on Membrane Progesterone (P4) Receptors Gene Expression in Bovine Myometrial Cells.Animals (Basel). 2022 Feb 19;12(4):519. doi: 10.3390/ani12040519. Animals (Basel). 2022. PMID: 35203226 Free PMC article.
References
-
- Robinson RS, Hammond AJ, Wathes DC, Hunter MG, Mann GE. Corpus luteum-endometrium-embryo interactions in the dairy cow: underlying mechanisms and clinical relevance. Reprod Domest Anim. 2008;43(Suppl 2):104–112. - PubMed
-
- Demetrio DG, Santos RM, Demetrio CG, Vasconcelos JL. Factors affecting conception rates following artificial insemination or embryo transfer in lactating Holstein cows. J Dairy Sci. 2007;90:5073–5082. - PubMed
-
- Green MP, Hunter MG, Mann GE. Relationships between maternal hormone secretion and embryo development on day 5 of pregnancy in dairy cows. Anim Reprod Sci. 2005;88:179–189. - PubMed
-
- Mann GE, Fray MD, Lamming GE. Effects of time of progesterone supplementation on embryo development and interferon-tau production in the cow. Vet J. 2006;171:500–503. - PubMed
-
- McNeill RE, Diskin MG, Sreenan JM, Morris DG. Associations between milk progesterone concentration on different days and with embryo survival during the early luteal phase in dairy cows. Theriogenology. 2006;65:1435–1441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

