Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation
- PMID: 22958438
- PMCID: PMC3462714
- DOI: 10.1186/1742-2094-9-212
Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation
Abstract
Background: In addition to systemic inflammation, neuroinflammation in the brain, which enhances sympathetic drive, plays a significant role in cardiovascular diseases, including hypertension. Oxidative stress in rostral ventrolateral medulla (RVLM) that augments sympathetic outflow to blood vessels is involved in neural mechanism of hypertension. We investigated whether neuroinflammation and oxidative stress in RVLM contribute to hypertension following chronic systemic inflammation.
Methods: In normotensive Sprague-Dawley rats, systemic inflammation was induced by infusion of Escherichia coli lipopolysaccharide (LPS) into the peritoneal cavity via an osmotic minipump. Systemic arterial pressure and heart rate were measured under conscious conditions by the non-invasive tail-cuff method. The level of the inflammatory markers in plasma or RVLM was analyzed by ELISA. Protein expression was evaluated by Western blot or immunohistochemistry. Tissue level of superoxide anion (O(2)(-)) in RVLM was determined using the oxidation-sensitive fluorescent probe dihydroethidium. Pharmacological agents were delivered either via infusion into the cisterna magna with an osmotic minipump or microinjection bilaterally into RVLM.
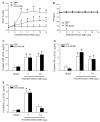
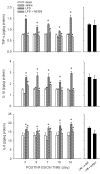
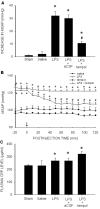
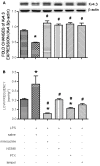
Results: Intraperitoneal infusion of LPS (1.2 mg/kg/day) for 14 days promoted sustained hypertension and induced a significant increase in plasma level of C-reactive protein, tumor necrosis factor-α (TNF-α), or interleukin-1β (IL-1β). This LPS-induced systemic inflammation was accompanied by activation of microglia, augmentation of IL-1β, IL-6, or TNF-α protein expression, and O(2)(-) production in RVLM, all of which were blunted by intracisternal infusion of a cycloxygenase-2 (COX-2) inhibitor, NS398; an inhibitor of microglial activation, minocycline; or a cytokine synthesis inhibitor, pentoxifylline. Neuroinflammation in RVLM was also associated with a COX-2-dependent downregulation of endothelial nitric oxide synthase and an upregulation of intercellular adhesion molecule-1. Finally, the LPS-promoted long-term pressor response and the reduction in expression of voltage-gated potassium channel, Kv4.3 in RVLM were antagonized by minocycline, NS398, pentoxifylline, or a superoxide dismutase mimetic, tempol, either infused into cisterna magna or microinjected bilaterally into RVLM. The same treatments, on the other hand, were ineffective against LPS-induced systemic inflammation.
Conclusion: These results suggest that systemic inflammation activates microglia in RVLM to induce COX-2-dependent neuroinflammation that leads to an increase in O(2)(-) production. The resultant oxidative stress in RVLM in turn mediates neurogenic hypertension.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

