FLT3-ITD knockin impairs hematopoietic stem cell quiescence/homeostasis, leading to myeloproliferative neoplasm
- PMID: 22958930
- PMCID: PMC3725984
- DOI: 10.1016/j.stem.2012.05.027
FLT3-ITD knockin impairs hematopoietic stem cell quiescence/homeostasis, leading to myeloproliferative neoplasm
Abstract
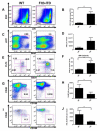
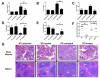
Internal tandem duplication (ITD) mutations within the FMS-like tyrosine kinase-3 (FLT3) render the receptor constitutively active driving proliferation and survival in leukemic blasts. Expression of FLT3-ITD from the endogenous promoter in a murine knockin model results in progenitor expansion and a myeloproliferative neoplasm. In this study, we show that this expansion begins with overproliferation within a compartment of normally quiescent long-term hematopoietic stem cells (LT-HSCs), which become rapidly depleted. This depletion is reversible upon treatment with the small molecule inhibitor Sorafenib, which also ablates the disease. Although the normal LT-HSC has been defined as FLT3(-) by flow cytometric detection, we demonstrate that FLT3 is capable of playing a role within this compartment by examining the effects of constitutively activated FLT3-ITD. This indicates an important link between stem cell quiescence/homeostasis and myeloproliferative disease while also giving novel insight into the emergence of FLT3-ITD mutations in the evolution of leukemic transformation.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures





References
-
- Adolfsson J, Borge OK, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, et al. Upregulation of FLT3 expression within the bone marrow Lin(−)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–69. - PubMed
-
- Akala OO, Clarke MF. Hematopoietic stem cell self-renewal. Curr Opin Genet Dev. 2008;16:496–501. - PubMed
-
- Auclair D, M. D, Yatsula V, Pickett W, Carter C, Chang Y, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. 2007;21:439–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

