Move in for the kill: motile microtubule regulators
- PMID: 22959403
- PMCID: PMC3482944
- DOI: 10.1016/j.tcb.2012.08.003
Move in for the kill: motile microtubule regulators
Abstract
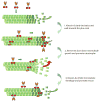
The stereotypical function of kinesin superfamily motors is to transport cargo along microtubules. However, some kinesins also shape the microtubule track by regulating microtubule assembly and disassembly. Recent work has shown that the kinesin-8 family of motors emerge as key regulators of cellular microtubule length. The studied kinesin-8s are highly processive motors that walk towards the microtubule plus-end. Once at plus-ends, they have complex effects on polymer dynamics; kinesin-8s either destabilize or stabilize microtubules, depending on the context. This review focuses on the mechanisms underlying kinesin-8-microtubule interactions and microtubule length control. We compare and contrast kinesin-8s with the other major microtubule-regulating kinesins (kinesin-4 and kinesin-13), to survey the current understanding of the diverse ways that kinesins control microtubule dynamics.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

References
-
- Maccioni RB, Cambiazo V. Role of microtubule-associated proteins in the control of microtubule assembly. Physiol Rev. 1995;75:835–864. - PubMed
-
- Gouveia SM, Akhmanova A. Cell and molecular biology of microtubule plus end tracking proteins: end binding proteins and their partners. Int Rev Cell Mol Biol. 2010;285:1–74. - PubMed
-
- Schuyler SC, Pellman D. Microtubule “plus-end-tracking proteins”: The end is just the beginning. Cell. 2001;105:421–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

