The prenyl-binding protein PrBP/δ: a chaperone participating in intracellular trafficking
- PMID: 22960045
- PMCID: PMC3514561
- DOI: 10.1016/j.visres.2012.08.013
The prenyl-binding protein PrBP/δ: a chaperone participating in intracellular trafficking
Abstract
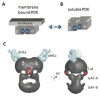
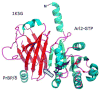
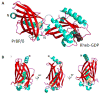
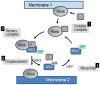
Expressed ubiquitously, PrBP/δ functions as chaperone/co-factor in the transport of a subset of prenylated proteins. PrBP/δ features an immunoglobulin-like β-sandwich fold for lipid binding, and interacts with diverse partners. PrBP/δ binds both C-terminal C15 and C20 prenyl side chains of phototransduction polypeptides and small GTP-binding (G) proteins of the Ras superfamily. PrBP/δ also interacts with the small GTPases, ARL2 and ARL3, which act as release factors (GDFs) for prenylated cargo. Targeted deletion of the mouse Pde6d gene encoding PrBP/δ resulted in impeded trafficking to the outer segments of GRK1 and cone PDE6 which are predicted to be farnesylated and geranylgeranylated, respectively. Rod and cone transducin trafficking was largely unaffected. These trafficking defects produce progressive cone-rod dystrophy in the Pde6d(-/-) mouse.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures




References
-
- Anant JS, Ong OC, Xie H, Clarke S, O’Brien PJ, Fung BKK. In vivo differential prenylation of retinal cyclic GMP phosphodiesterase catalytic subunits. J Biol Chem. 1992;267:687–690. - PubMed
-
- Aspuria PJ, Tamanoi F. The Rheb family of GTP-binding proteins. Cell Signal. 2004;16:1105–1112. - PubMed
-
- Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. - PubMed
-
- Baehr W, Devlin MJ, Applebury ML. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979;254:11669–11677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

