The glucagon-like peptide-1 receptor agonist Exendin 4 has a protective role in ischemic injury of lean and steatotic liver by inhibiting cell death and stimulating lipolysis
- PMID: 22960075
- PMCID: PMC5691335
- DOI: 10.1016/j.ajpath.2012.07.015
The glucagon-like peptide-1 receptor agonist Exendin 4 has a protective role in ischemic injury of lean and steatotic liver by inhibiting cell death and stimulating lipolysis
Abstract
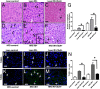
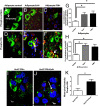
Nonalcoholic fatty liver disease is an increasingly prevalent spectrum of conditions characterized by excess fat deposition within hepatocytes. Affected hepatocytes are known to be highly susceptible to ischemic insults, responding to injury with increased cell death, and commensurate liver dysfunction. Numerous clinical circumstances lead to hepatic ischemia. Mechanistically, specific means of reducing hepatic vulnerability to ischemia are of increasing clinical importance. In this study, we demonstrate that the glucagon-like peptide-1 receptor agonist Exendin 4 (Ex4) protects hepatocytes from ischemia reperfusion injury by mitigating necrosis and apoptosis. Importantly, this effect is more pronounced in steatotic livers, with significantly reducing cell death and facilitating the initiation of lipolysis. Ex4 treatment leads to increased lipid droplet fission, and phosphorylation of perilipin and hormone sensitive lipase - all hallmarks of lipolysis. Importantly, the protective effects of Ex4 are seen after a short course of perioperative treatment, potentially making this clinically relevant. Thus, we conclude that Ex4 has a role in protecting lean and fatty livers from ischemic injury. The rapidity of the effect and the clinical availability of Ex4 make this an attractive new therapeutic approach for treating fatty livers at the time of an ischemic insult.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Mitigation of autophagy ameliorates hepatocellular damage following ischemia-reperfusion injury in murine steatotic liver.Am J Physiol Gastrointest Liver Physiol. 2014 Dec 1;307(11):G1088-99. doi: 10.1152/ajpgi.00210.2014. Epub 2014 Sep 25. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 25258410 Free PMC article.
-
Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis.Liver Int. 2011 Oct;31(9):1285-97. doi: 10.1111/j.1478-3231.2011.02462.x. Epub 2011 Feb 15. Liver Int. 2011. PMID: 21745271
-
GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy.PLoS One. 2011;6(9):e25269. doi: 10.1371/journal.pone.0025269. Epub 2011 Sep 21. PLoS One. 2011. PMID: 21957486 Free PMC article.
-
Reactive oxygen and nitrogen species in steatotic hepatocytes: a molecular perspective on the pathophysiology of ischemia-reperfusion injury in the fatty liver.Antioxid Redox Signal. 2014 Sep 1;21(7):1119-42. doi: 10.1089/ars.2013.5486. Epub 2014 Feb 19. Antioxid Redox Signal. 2014. PMID: 24294945 Free PMC article. Review.
-
Apoptosis and necrosis in the liver.Compr Physiol. 2013 Apr;3(2):977-1010. doi: 10.1002/cphy.c120020. Compr Physiol. 2013. PMID: 23720337 Free PMC article. Review.
Cited by
-
Effects of glucagon-like peptide-1 receptor agonists on non-alcoholic fatty liver disease and inflammation.World J Gastroenterol. 2014 Oct 28;20(40):14821-30. doi: 10.3748/wjg.v20.i40.14821. World J Gastroenterol. 2014. PMID: 25356042 Free PMC article. Review.
-
Modulation of Prostanoids Profile and Counter-Regulation of SDF-1α/CXCR4 and VIP/VPAC2 Expression by Sitagliptin in Non-Diabetic Rat Model of Hepatic Ischemia-Reperfusion Injury.Int J Mol Sci. 2021 Dec 5;22(23):13155. doi: 10.3390/ijms222313155. Int J Mol Sci. 2021. PMID: 34884960 Free PMC article.
-
Circulating concentrations of GLP-1 are associated with coronary atherosclerosis in humans.Cardiovasc Diabetol. 2013 Aug 16;12:117. doi: 10.1186/1475-2840-12-117. Cardiovasc Diabetol. 2013. PMID: 23953602 Free PMC article.
-
Consensus Recommendations on GLP-1 RA Use in the Management of Type 2 Diabetes Mellitus: South Asian Task Force.Diabetes Ther. 2019 Oct;10(5):1645-1717. doi: 10.1007/s13300-019-0669-4. Epub 2019 Jul 29. Diabetes Ther. 2019. PMID: 31359367 Free PMC article. Review.
-
Exenatide regulates pancreatic islet integrity and insulin sensitivity in the nonhuman primate baboon Papio hamadryas.JCI Insight. 2019 Oct 17;4(20):e93091. doi: 10.1172/jci.insight.93091. JCI Insight. 2019. PMID: 31536476 Free PMC article.
References
-
- Hanouneh I.A., Zein N.N. Metabolic syndrome and liver transplantation. Minerva Gastroenterol Dietol. 2010;56:297–304. - PubMed
-
- Selzner M., Clavien P.A. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–113. - PubMed
-
- Gomez D., Malik H.Z., Bonney G.K., Wong V., Toogood G.J., Lodge J.P., Prasad K.R. Steatosis predicts postoperative morbidity following hepatic resection for colorectal metastasis. Br J Surg. 2007;94:1395–1402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

