Flux of ionic dyes across microneedle-treated skin: effect of molecular characteristics
- PMID: 22960319
- PMCID: PMC4119965
- DOI: 10.1016/j.ijpharm.2012.08.026
Flux of ionic dyes across microneedle-treated skin: effect of molecular characteristics
Abstract
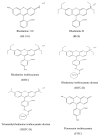
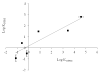
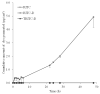
Drug flux across microneedle (MN)-treated skin is influenced by the characteristics of the MN array, formed microconduits and physicochemical properties of the drug molecules in addition to the overall diffusional resistance of microconduits and viable tissue. Relative implication of these factors has not been fully explored. In the present study, the in vitro permeation of a series of six structurally related ionic xanthene dyes with different molecular weights (MW) and chemical substituents, across polymer MN-pretreated porcine skin was investigated in relation of their molecular characteristics. Dyes equilibrium solubility, partition coefficient in both n-octanol or porcine skin/aqueous system, and dissociation constants were determined. Results indicated that for rhodamine dyes, skin permeation of the zwitterionic form which predominates at physiological pH, was significantly reduced by an increase in MW, the skin thickness and by the presence of the chemically reactive isothiocyanate substituent. These factors were generally shown to override the aqueous solubility, an important determinant of drug diffusion in an aqueous milieu. The data obtained provided more insight into the mechanism of drug permeation across MN-treated skin, which is of importance to both the design of MN-based transdermal drug delivery systems and of relevance to skin permeation research.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures




Similar articles
-
Microneedle/nanoencapsulation-mediated transdermal delivery: mechanistic insights.Eur J Pharm Biopharm. 2014 Feb;86(2):145-55. doi: 10.1016/j.ejpb.2013.01.026. Epub 2013 Feb 24. Eur J Pharm Biopharm. 2014. PMID: 23461860 Free PMC article.
-
Optical coherence tomography is a valuable tool in the study of the effects of microneedle geometry on skin penetration characteristics and in-skin dissolution.J Control Release. 2010 Nov 1;147(3):333-41. doi: 10.1016/j.jconrel.2010.08.008. Epub 2010 Aug 18. J Control Release. 2010. PMID: 20727929
-
Vehicle composition influence on the microneedle-enhanced transdermal flux of naltrexone hydrochloride.Pharm Res. 2011 Jan;28(1):124-34. doi: 10.1007/s11095-010-0191-x. Epub 2010 Jun 25. Pharm Res. 2011. PMID: 20577787
-
Microneedle-Assisted Percutaneous Transport of Magnesium Sulfate.Curr Drug Deliv. 2020;17(2):140-147. doi: 10.2174/1567201817666191217093936. Curr Drug Deliv. 2020. PMID: 31845631
-
Current aspects of formulation efforts and pore lifetime related to microneedle treatment of skin.Expert Opin Drug Deliv. 2010 May;7(5):617-29. doi: 10.1517/17425241003663228. Expert Opin Drug Deliv. 2010. PMID: 20205604 Free PMC article. Review.
Cited by
-
Mathematical Modelling, Simulation and Optimisation of Microneedles for Transdermal Drug Delivery: Trends and Progress.Pharmaceutics. 2020 Jul 22;12(8):693. doi: 10.3390/pharmaceutics12080693. Pharmaceutics. 2020. PMID: 32707878 Free PMC article. Review.
-
Microneedle/nanoencapsulation-mediated transdermal delivery: mechanistic insights.Eur J Pharm Biopharm. 2014 Feb;86(2):145-55. doi: 10.1016/j.ejpb.2013.01.026. Epub 2013 Feb 24. Eur J Pharm Biopharm. 2014. PMID: 23461860 Free PMC article.
-
Microneedle-Assisted Transfersomes as a Transdermal Delivery System for Aspirin.Pharmaceutics. 2023 Dec 29;16(1):57. doi: 10.3390/pharmaceutics16010057. Pharmaceutics. 2023. PMID: 38258069 Free PMC article.
-
A 3D-printed microfluidic-enabled hollow microneedle architecture for transdermal drug delivery.Biomicrofluidics. 2019 Dec 11;13(6):064125. doi: 10.1063/1.5127778. eCollection 2019 Nov. Biomicrofluidics. 2019. PMID: 31832123 Free PMC article.
-
Characterisation of Drug Delivery Efficacy Using Microstructure-Assisted Application of a Range of APIs.Pharmaceutics. 2020 Dec 15;12(12):1213. doi: 10.3390/pharmaceutics12121213. Pharmaceutics. 2020. PMID: 33333795 Free PMC article.
References
-
- Donnelly RF, Singh TRR, Morrow DIJ, Woolfson AD. Microneedle-mediated transdermal and intradermal drug delivery. John Wiley & Sons, Ltd; Oxford, UK: 2012.
-
- Milewski M, Stinchcomb AL. Vehicle composition influence on the microneedle-enhanced transdermal flux of naltrexone hydrochloride. Pharm Res. 2011;28(1):124–134. - PubMed
-
- Guy RH, Hadgraft J. Physicochemical aspects of percutaneous penetration and its enhancement. Pharm Res. 1988;5(12):753–8. - PubMed
-
- Bariya SH, Gohel MC, Mehta TA, Sharma OP. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64(1):11–29. - PubMed
-
- Teo MA, Shearwood C, Ng KC, Lu J, Moochhala S. In vitro and in vivo characterization of MEMS microneedles. Biomed Microdevices. 2005;7(1):47–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

