Phosphatidylethanolamine is externalized at the surface of microparticles
- PMID: 22960380
- PMCID: PMC3809829
- DOI: 10.1016/j.bbalip.2012.08.017
Phosphatidylethanolamine is externalized at the surface of microparticles
Abstract
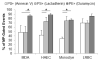
Microparticles (MPs) are membrane-bound vesicles shed normally or as a result of various (pathological) stimuli. MPs contain a wealth of bio-active macromolecules. Aminophospholipid phosphatidylserine (PS) is present on the surface of many MPs. As PS and phosphatidylethanolamine (PE) are related, yet distinct aminophospholipids, the purpose of this study was to systematically and directly assess PE exposure on MPs. We examined MPs from various human cellular sources (human breast cancer, endothelial, red and white blood cells) by flow cytometry using a PE-specific probe, duramycin, and two PS-specific probes, annexin V and lactadherin. PS and PE exposure percentage was comparable on vascular and blood cell-derived MPs (80-90% of MP-gated events). However, the percentage of malignant breast cancer MPs exposing PE (~90%) was significantly higher than PS (~50%). Thus, while PS and PE exposure can result from a general loss of membrane asymmetry, there may also be distinct mechanisms of PE and PS exposure on MPs that vary by cellular source.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures


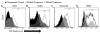


References
-
- Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010;107:1047–1057. - PubMed
-
- Amabile N, Rautou P-E, Tedgui A, Boulanger CM. Microparticles: key protagonists in cardiovascular disorders. Semin. Thromb. Hemost. 2010;36:907–916. - PubMed
-
- Tushuizen ME, Diamant M, Sturk A, Nieuwland R. Cell-derived microparticles in the pathogenesis of cardiovascular disease: friend or foe? Arterioscler. Thromb. Vasc. Biol. 2011;31:4–9. - PubMed
-
- Rak J. Microparticles in cancer. Semin. Thromb. Hemost. 2010;36:888–906. - PubMed
-
- Zwicker JI. Predictive value of tissue factor bearing microparticles in cancer associated thrombosis. Thromb. Res. 2010;125(Suppl 2):S89–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

