Perinatal exposure to 50 ppb sodium arsenate induces hypothalamic-pituitary-adrenal axis dysregulation in male C57BL/6 mice
- PMID: 22960421
- PMCID: PMC3470737
- DOI: 10.1016/j.neuro.2012.08.010
Perinatal exposure to 50 ppb sodium arsenate induces hypothalamic-pituitary-adrenal axis dysregulation in male C57BL/6 mice
Abstract
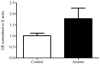
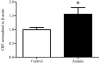
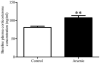
Over the past two decades, key advancements have been made in understanding the complex pathology that occurs following not only high levels of arsenic exposure (>1 ppm) but also levels previously considered to be low (<100 ppb). Past studies have characterized the deleterious effects of arsenic on the various functions of cardiovascular, pulmonary, immunological, respiratory, endocrine and neurological systems. Other research has demonstrated an elevated risk of a multitude of cancers and increased rates of psychopathology, even at very low levels of arsenic exposure. The hypothalamic-pituitary-adrenal (HPA) axis represents a multisite integration center that regulates a wide scope of biological and physiological processes: breakdown within this system can generate an array of far-reaching effects, making it an intriguing candidate for arsenic-mediated damage. Using a mouse model, we examined the effects of perinatal exposure to 50 ppb sodium arsenate on the functioning of the HPA axis through the assessment of corticotrophin-releasing factor (CRF), proopiomelanocortin (Pomc) mRNA, adrenocorticotrophin hormone (ACTH), corticosterone (CORT), 11β-hydroxysteroid dehydrogenase Type 1 (11β-HSD 1), and glucocorticoid receptor (GR) protein and mRNA. Compared to controls, we observed that the perinatal arsenic-exposed offspring exhibit an increase in hypothalamic CRF, altered CORT secretion both at baseline and in response to a stressor, decreased hippocampal 11β-HSD 1 and altered subcellular GR distribution in the hypothalamus. These data indicate significant HPA axis impairment at post-natal day 35 resulting from perinatal exposure to 50 ppb sodium arsenate. Our findings suggest that the dysregulation of this critical regulatory axis could underlie important molecular and cognitive pathology observed following exposure to arsenic.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures







References
-
- ATSDR . Toxicological Profile for Arsenic (Update) Agency for Toxic Substances and Disease Registry, SUDHHS, PHS; Washington, DC: 1999. - PubMed
-
- Blum A, Martin HJ, Maser E. Human 11beta-hydroxysteroid dehydrogenase type 1 is enzymatically active in its nonglycosylated form. Biochem Biophys Res Commun. 2000;276(2):428–34. - PubMed
-
- Buckley CT, Caldwell KK. Fear conditioning is associated with altered integration of PLC and ERK signaling in the hippocampus. Pharmacol Biochem Behav. 2004;79:633–40. - PubMed
-
- Bodwell JE, Kingsley LA, Hamilton JW. Arsenic at very low concentrations alters glucocorticoid receptor (GR) –mediated gene activation but not GR-mediated gene repression: complex dose-response effects are closely correlated with levels of activated GR and require a functional GR DNA binding domain. Chem Res Toxicol. 2004;17:1064–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

