Isolation of Chlamydia trachomatis and membrane vesicles derived from host and bacteria
- PMID: 22960504
- PMCID: PMC3492957
- DOI: 10.1016/j.mimet.2012.08.012
Isolation of Chlamydia trachomatis and membrane vesicles derived from host and bacteria
Abstract
The study of intracellular bacteria and nanometer-size membrane vesicles within infected host cells poses an important challenge as it is difficult to identify each distinct population in the context of the complex populations generated from active host-pathogen interactions. Here, suspension cultures of L929 cells infected with the prevalent obligate intracellular bacterium Chlamydia trachomatis strain F/Cal-IC-13 are utilized for the large scale preparation and isolation of natural membrane vesicles and bacterial forms. Cell lysis with nitrogen cavitation in combination with differential centrifugation, OptiPrep™ density gradient separation, and immunoenrichment using anti-chlamydial lipopolysaccharide antibodies and MagnaBind beads allows for the isolation of both productive and persistent bacterial forms, as well as membrane vesicles derived from the host and pathogen. We have evaluated these populations by electron microscopy and Western blot analysis for identification of biomarkers. In addition, purified persistent forms of C. trachomatis induced by ampicillin display adenosine-5'-triphosphate (ATP) transport activity, suggesting that ampicillin-induced persistent C. trachomatis organisms, at least in part, rely upon host ATP as an energy source. Importantly, several chlamydial cytotoxic and/or secreted proteins are demonstrated to be associated with these vesicles, supporting the idea that membrane vesicles are generated by Chlamydia as a means of carrying and delivering virulence factors necessary for pathogenesis. The ability to produce large-scale infections and generate distinct bacteria and host-derived populations for biochemical analysis, while reducing the burdens of time and cost have implications in all areas of chlamydiology. These protocols can be applied to other strains of C. trachomatis or other intracellular bacteria.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures





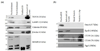
Similar articles
-
A meta-analysis of affinity purification-mass spectrometry experimental systems used to identify eukaryotic and chlamydial proteins at the Chlamydia trachomatis inclusion membrane.J Proteomics. 2020 Feb 10;212:103595. doi: 10.1016/j.jprot.2019.103595. Epub 2019 Nov 21. J Proteomics. 2020. PMID: 31760040 Free PMC article.
-
Pmp-like proteins Pls1 and Pls2 are secreted into the lumen of the Chlamydia trachomatis inclusion.Infect Immun. 2008 Sep;76(9):3940-50. doi: 10.1128/IAI.00632-08. Epub 2008 Jun 30. Infect Immun. 2008. PMID: 18591235 Free PMC article.
-
Dynamic energy dependency of Chlamydia trachomatis on host cell metabolism during intracellular growth: Role of sodium-based energetics in chlamydial ATP generation.J Biol Chem. 2018 Jan 12;293(2):510-522. doi: 10.1074/jbc.M117.797209. Epub 2017 Nov 9. J Biol Chem. 2018. PMID: 29123027 Free PMC article.
-
Membrane vesicle production by Chlamydia trachomatis as an adaptive response.Front Cell Infect Microbiol. 2014 Jun 10;4:73. doi: 10.3389/fcimb.2014.00073. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24959424 Free PMC article. Review.
-
Chlamydia trachomatis: the Persistent Pathogen.Clin Vaccine Immunol. 2017 Oct 5;24(10):e00203-17. doi: 10.1128/CVI.00203-17. Print 2017 Oct. Clin Vaccine Immunol. 2017. PMID: 28835360 Free PMC article. Review.
Cited by
-
Identification of Outer Membrane Vesicles Derived from Orientia tsutsugamushi.J Korean Med Sci. 2015 Jul;30(7):866-70. doi: 10.3346/jkms.2015.30.7.866. Epub 2015 Jun 10. J Korean Med Sci. 2015. PMID: 26130947 Free PMC article.
-
Human genital antibody-mediated inhibition of Chlamydia trachomatis infection and evidence for ompA genotype-specific neutralization.PLoS One. 2021 Oct 18;16(10):e0258759. doi: 10.1371/journal.pone.0258759. eCollection 2021. PLoS One. 2021. PMID: 34662351 Free PMC article.
-
Context-Dependent Action of Scc4 Reinforces Control of the Type III Secretion System.J Bacteriol. 2020 Jul 9;202(15):e00132-20. doi: 10.1128/JB.00132-20. Print 2020 Jul 9. J Bacteriol. 2020. PMID: 32424009 Free PMC article.
-
Extracellular Vesicles Could Carry an Evolutionary Footprint in Interkingdom Communication.Front Cell Infect Microbiol. 2020 Mar 3;10:76. doi: 10.3389/fcimb.2020.00076. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32195195 Free PMC article. Review.
-
Chlamydia trachomatis infection results in a modest pro-inflammatory cytokine response and a decrease in T cell chemokine secretion in human polarized endocervical epithelial cells.Cytokine. 2013 Aug;63(2):151-65. doi: 10.1016/j.cyto.2013.04.022. Epub 2013 May 11. Cytokine. 2013. PMID: 23673287 Free PMC article.
References
-
- Andersen SR, Bjune G, Hoiby EA, Michaelsen TE, Aase A, Rye U, Jantzen E. Outer membrane vesicle vaccines made from short-chain lipopolysaccharide mutants of serogroup B Neisseria meningitidis: effect of the carbohydrate chain length on the immune response. Vaccine. 1997;15:1225–1234. - PubMed
-
- Arigita C, Luijkx T, Jiskoot W, Poelen M, Hennink WE, Crommelin DJ, Ley P, Els C, Kersten GF. Well-defined and potent liposomal meningococcal B vaccines adjuvated with LPS derivatives. Vaccine. 2005;23:5091–5098. - PubMed
-
- Beatty WL. Trafficking from CD63-positive late endocytic multivesicular bodies is essential for intracellular development of Chlamydia trachomatis. J. Cell Sci. 2006;119:350–359. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

